Figure 4.
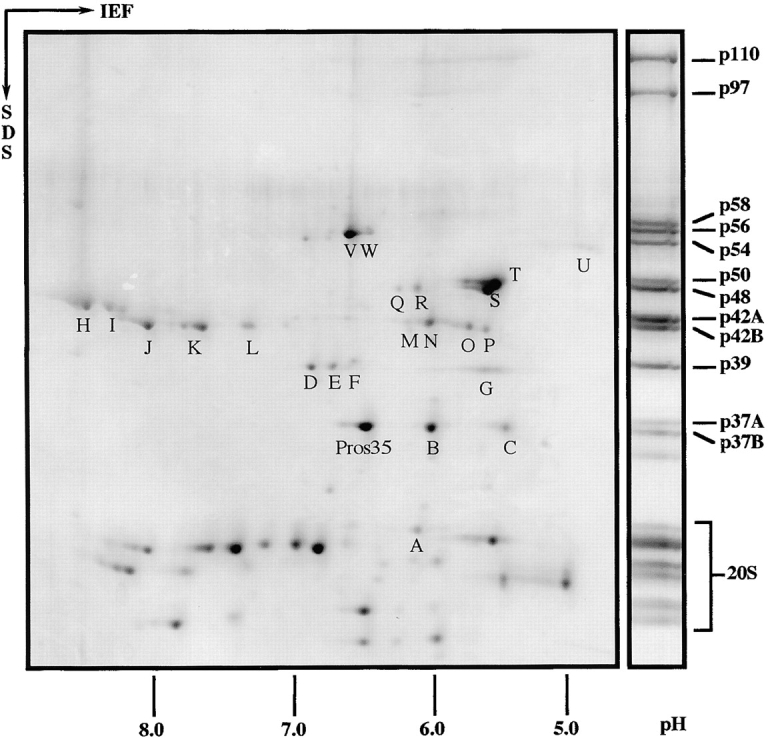
2D gel electrophoresis of Drosophila 26S proteasomes. Purified 26S proteasomes from Drosophila embryos were separated in the first dimension by isoelectric focusing (IEF) with an immobilized pH gradient from 3 to 10. Next, proteins were resolved in the second dimension using a 12.5% polyacrylamide SDS gel and stained with Coomassie blue. 26S proteasomes resolved by 1D SDS-PAGE were used as molecular weight marker and the bands were named according to the procedure of Haracska and Udvardy 1996. All spots marked with a capital were identified by peptide sequencing with the exception of spot L, which could not be sequenced, and spot U, which was identified by immunoblotting (for details see Table and Results). Low molecular weight proteins (not indicated separately) belong to the 20S core complex. With one exception, Pros35, they were not identified by peptide sequencing.
