Figure 7.
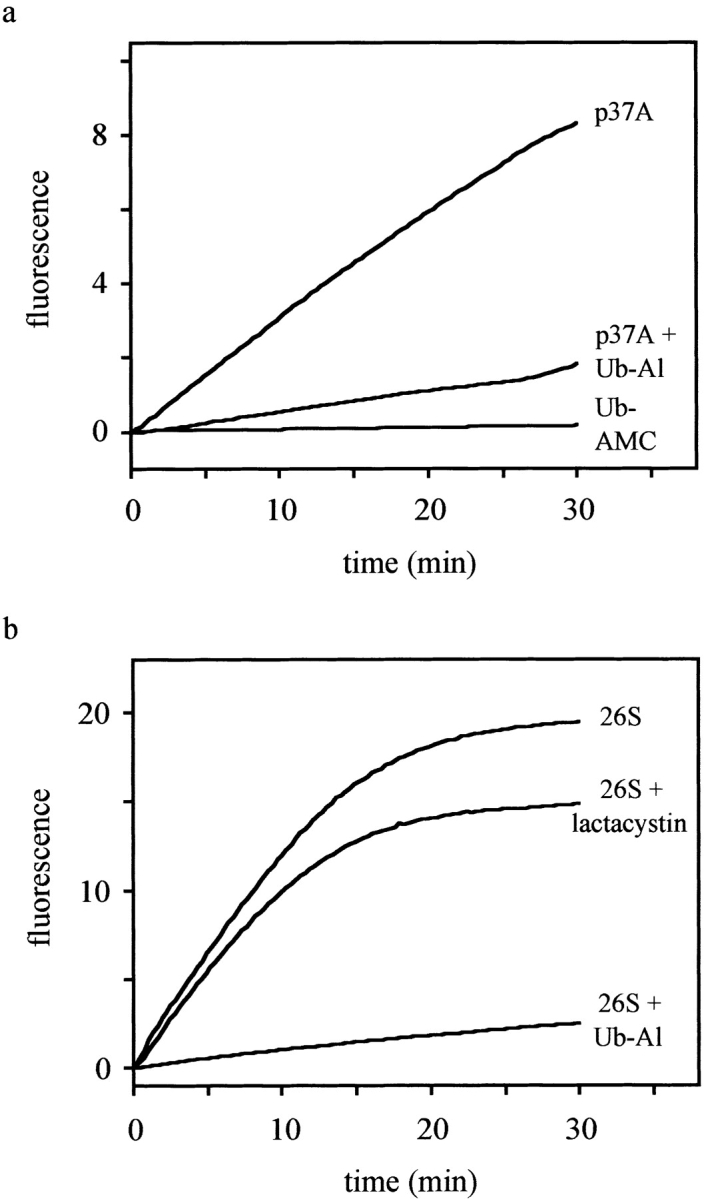
p37A and 26S proteasomes hydrolyze Ub-AMC. The fluorescence intensity (λex = 380 nm, λem = 460 nm), which is proportional to the release of AMC, was recorded and plotted as a function of time. The reactions were performed at 25°C in assay buffer (50 mM Hepes, 0.5 mM EDTA, pH 7.8, 1 mM DTT, and 0.1 mg/ml BSA) using Ub-AMC at a final concentration of 5 μM. a, The activity of p37A (∼2 nM) was measured both with and without preincubation with Ub-Al (2 nM). In a control assay, no enzyme was added to exclude self-hydrolysis of Ub-AMC. b, Similarly, 26S proteasomes (∼20 μg/ml) were preincubated with and without Ub-Al (10 nM), or with lactacystin (20 μM) before addition of Ub-AMC. The difference in the levels of fluorescence between uninhibited and lactacystin-inhibited proteasomes lies within the normally observed range of deviation.
