Fig. 1. Cryptosporidium parvum selectively recruits components of host-cell SEMs to infection sites.
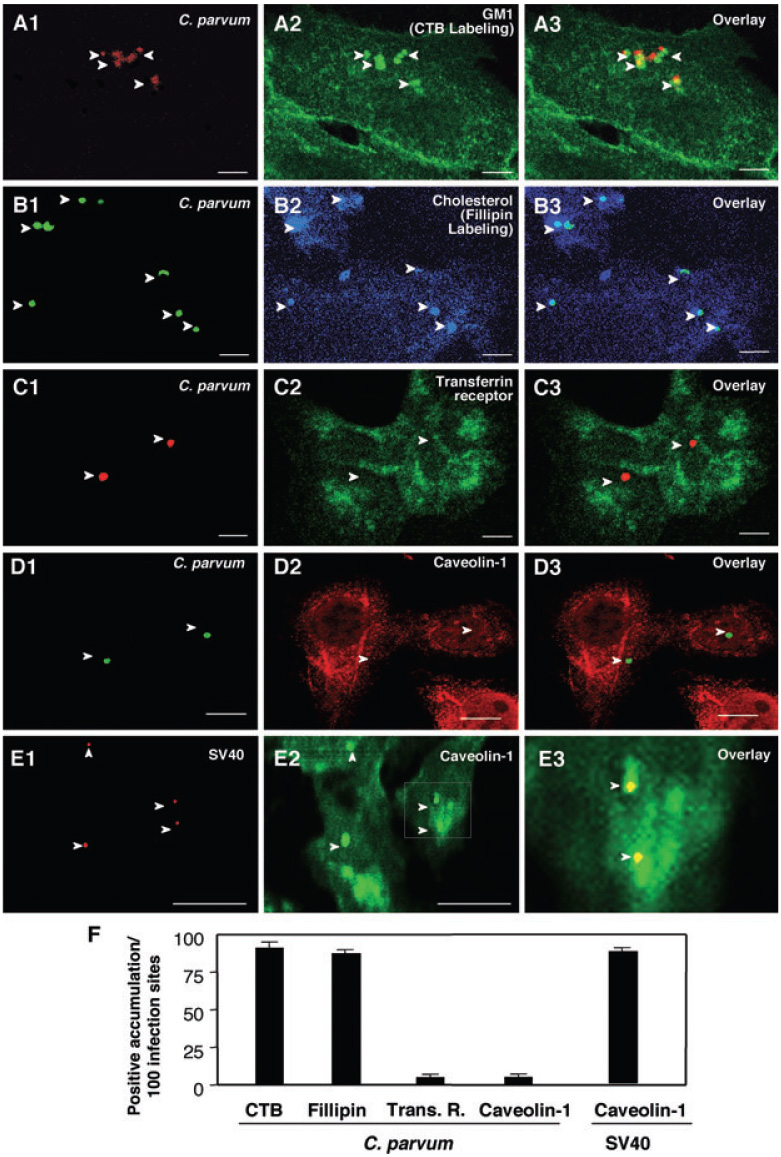
A–E. H69 cells were infected with C. parvum and labelled with various markers to identify SEMs. C. parvum was labelled using an specific antibody [arrowheads in (A1)–(D1)]. Accumulation of GM1 (labelled by cholera toxin B) and cholesterol (labelled by Fillipin), components of SEMs, was observed at infection sites [arrowheads in (A2) and (B2) or in the overlay images of (A3) and (B3)]. Transferrin receptor, a non-SEM-associated membrane receptor, showed no significant accumulation at infection sites ([arrowheads in (C2) and (C3)]. Moreover, caveolin-1, a protein that exists in some SEMs and is associated with caveolae, did not accumulate at infection sites [arrowheads in (D2) and (D3)]. As a positive control, a significant accumulation of caveolin-1 was detected at the infection sites of SV40 [arrowheads in (E1) and (E2)]. (E3) is an overlay image of high magnification of the boxed region in (E2). Bar = 5 µm.
F. Quantitative analysis as determined by number of sites with positive accumulation per 100 infection sites. CTB, cholera toxin B.
