Figure 6. AMPK directly phosphorylates FOXO/DAF-16 in vitro.
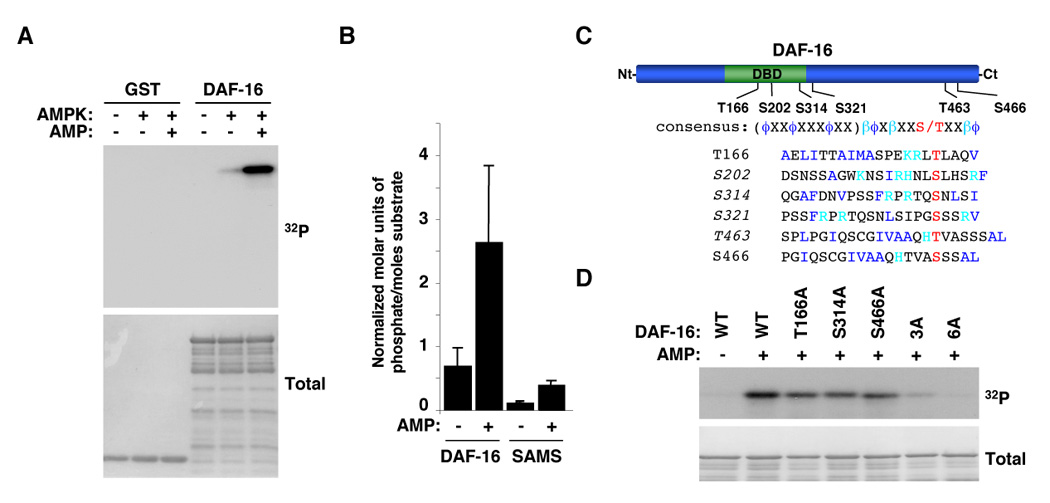
(A) AMPK phosphorylates DAF-16 in an AMP-dependent manner. Results with human AMPK are presented but both human and worm AMPK phosphorylate DAF-16 in an AMP-dependent manner. (B) Stoichiometry of phosphorylation of DAF-16 by human AMPK. The graph represents the mean and standard deviation of normalized molar units of phosphate incorporated per mole of substrate of 3 independent experiments. Similar results were obtained with the worm AMPK. (C) Schematic of DAF-16 showing the location of the residues of DAF-16 phosphorylated by AMPK in vitro. DBD: DNA binding domain. Alignment of the AMPK consensus phosphorylation motif with phosphorylation sites in DAF-16. ϕ: hydrophobic residues; β: basic residues. The sequence in parenthesis represents an amphipathic helix. The sites in italics were identified as being phosphorylated in vitro by AMPK by tandem mass spectrometry. All the sites listed here were confirmed as being phosphorylated in vitro by AMPK by site-directed mutagenesis. (D) DAF-16 mutants display a significant reduction in phosphorylation by AMPK compared to WT DAF-16 in an in vitro kinase assay. 3A: T166A/S314A/S466A mutant; 6A: T166A/S202A/S314A/S321A/T463A/S466A.
