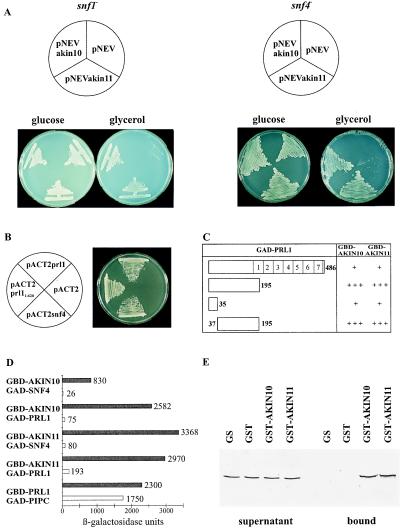
Figure 1.
AKIN10 and AKIN11 complement the snf1Δ10 deficiency, suppress the snf4Δ2 mutation, and interact with SNF4p and PRL1 in yeast. (A) Unlike controls with the empty vector pNEV, yeast strains MCY1846 snf1Δ10 (Left) and MCY1853 snf4Δ2 (Right), carrying pNEVakin10 and pNEVakin11, grew equally well on glucose and glycerol. (B) Interaction of GBD-SNF1p with GAD-PRL1 and GAD-SNF4p. Yeast carrying pAS1snf1 was transformed with pACT2prl1, pACT2prl11–620, pACT2snf4, and pACT2 as control and grew on SD medium in the presence of 50 mM 3-aminotriazole. (C) Mapping of PRL1 domain involved in binding of AKIN10 and AKIN11. GBD-AKIN10 and GBD-AKIN11 were combined with GAD-PRL1 and GAD fusions of PRL1 peptides located between positions 1 and 35, 1 and 195, and 37 and 195. LacZ-filter lift assays were performed with yeast colonies grown on SC medium with 2% glucose for 3 days at 30°C. In open bars symbolizing the GAD-PRL1 constructs (open bars), numbers indicate the boundaries of deletions and WD-40 repeats. 5-Bromo-4-chloro-3-indolyl β-d-galactoside staining after 4 h is marked by +++, whereas weaker staining observed after 16 h is labeled by +. (D) Glucose regulation of protein interactions of AKIN10 and AKIN11 with SNF4p and PRL1 in yeast. GBD-AKIN10 and GBD-AKIN11 were combined with either GAD-SNF4 or GAD-PRL1 in yeast Y190 grown either in SC medium with 2% glucose (open bars) or in SC-Gal/Gly/EtOH medium with 0.05% glucose (solid bars). β-Galactosidase activities were determined by using an o-nitrophenylgalactosidase assay (15). As control, interaction of GBD-PRL1 with the PRL1-binding protein GAD-PIPC was assayed similarly. The standard deviation of each assay was less than 10% of the maximum value measured. (E) Interaction of PRL1 with GST-AKIN10 and GST-AKIN11 in vitro. 35S-labeled PRL1 was incubated with immobilized GST, GST-AKIN10, GST-AKIN11, and control glutathione-Sepharose 4B matrix. The supernatant (Left) and matrix-bound (Right) fractions were separated by SDS/PAGE to detect the labeled PRL1 protein by autoradiography.