Figure 2.
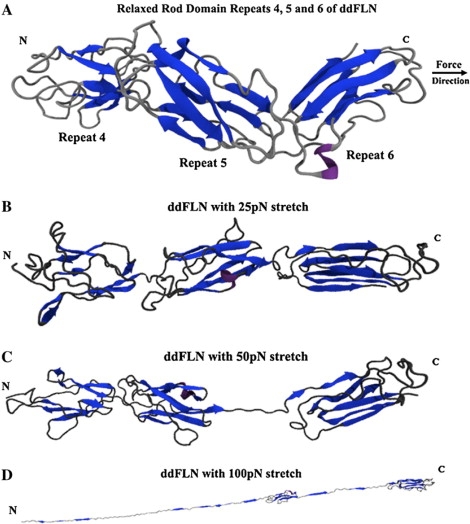
(A) The native conformation of Dictyostelium discoideum's rod domain repeats 4, 5, and 6. Force is applied to the C-terminal α-carbon away from the N-terminal α-carbon. This force approximates the physiological effect of stretching on the rod domain of filamin molecules. Note the staggered conformation and tertiary structure of the interrepeat region, which will be rapidly distorted and unfolded with force application. In addition, repeat 4 is in its closed state, concealing its core. (B) Within 30 ps at 25 pN of force, the staggered geometry is lost within the rod domain of ddFLN. Regions of the protein begin to become distorted: notice the strained geometry identified as a helical loop in repeat 5 and that the interrepeat of 5 and 6 begins to unfold. The repeats are aligned in line with the axis of the applied force. (C) After a minimum of 100 ps at 50 pN, the predicted weak linker region between repeats 5 and 6 unfolds completely. Unfolding this region can allow the rest of the rod domain to move more freely, whereas the dimer holds together tightly, and actin is still bound through ABDs. (D) Force >100 pN is required to unfold repeat 6. Repeat 5 still contains some detectable tertiary structure, but the majority of its β-strands have been pulled out of coordination. At 75 pN the linker region between repeats 4 and 5 is intact and unfolds only when the repeats themselves begin to lose large degrees of structure.
