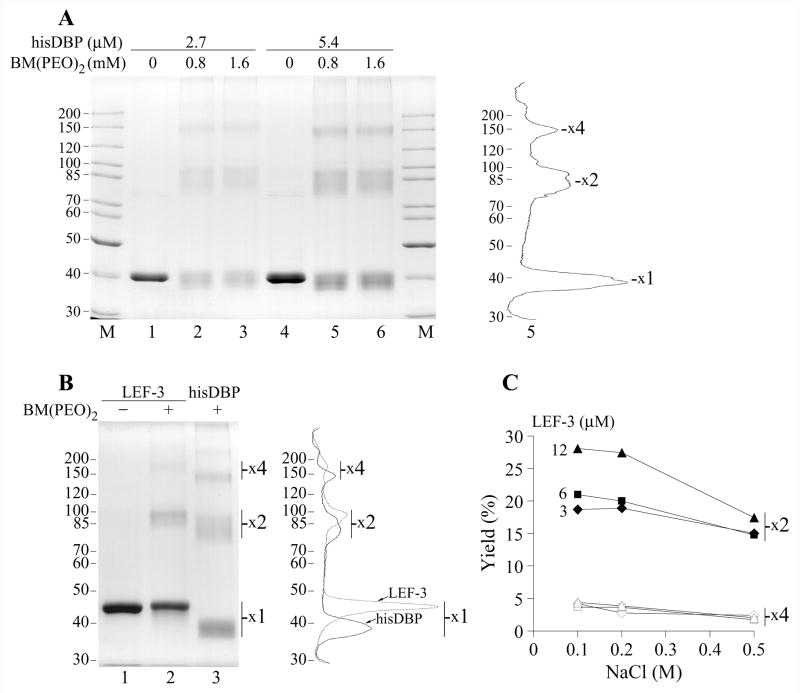
Figure 4.
Crosslinking of DBP and LEF-3 by BM(PEO)2. (A) The his-tagged DBP at concentrations of 2.7 μM (lanes 1–3) or 5.4 μM (lanes 4–6) was incubated in 12-μl mixtures containing 50 mM Tris-HCl, pH 7.5, 0.2 M NaCl, 25% glycerol, in the absence of the crosslinker (lanes 1 and 4) or in the presence of BM(PEO)2 at concentrations of 0.8 mM (lanes 2 and 5) and 1.6 mM (lanes 3 and 6). After incubation for 1 h at room temperature, the crosslinking reaction was terminated by addition of 40 mM DTT and the samples were analyzed by SDS-8% PAGE. Densitometry of lane 5 is shown on the right. (B) LEF-3 at concentration of 4.5 μM was incubated in 10-μl mixtures containing 50 mM Tris-HCl, pH 7.5, 0.2 M NaCl, 13% glycerol, in the absence of the crosslinker (lane 1) or in the presence of 1 mM BM(PEO)2 (lane 2) for 1 h at room temperature. Lane 3 shows hisDBP (4.5 μM) crosslinked by BM(PEO)2 at the same conditions. The samples were analyzed by SDS-8% PAGE. Positions of putative protein monomers (x1), dimers (x2) and tetramers (x4) are indicated. Densitometry of lanes 2 and 3 is shown on the right. (C) Yield of protein dimers and tetramers after crosslinking of LEF-3 at concentration 3, 6, and 12 μM in the presence of NaCl varied from 0.1 to 0.5 M. LEF-3 was crosslinked by 1 mM BM(PEO)2 and analyzed as in panel B. Closed and open symbols show respectively the dimers and tetramers at indicated concentrations of LEF-3.