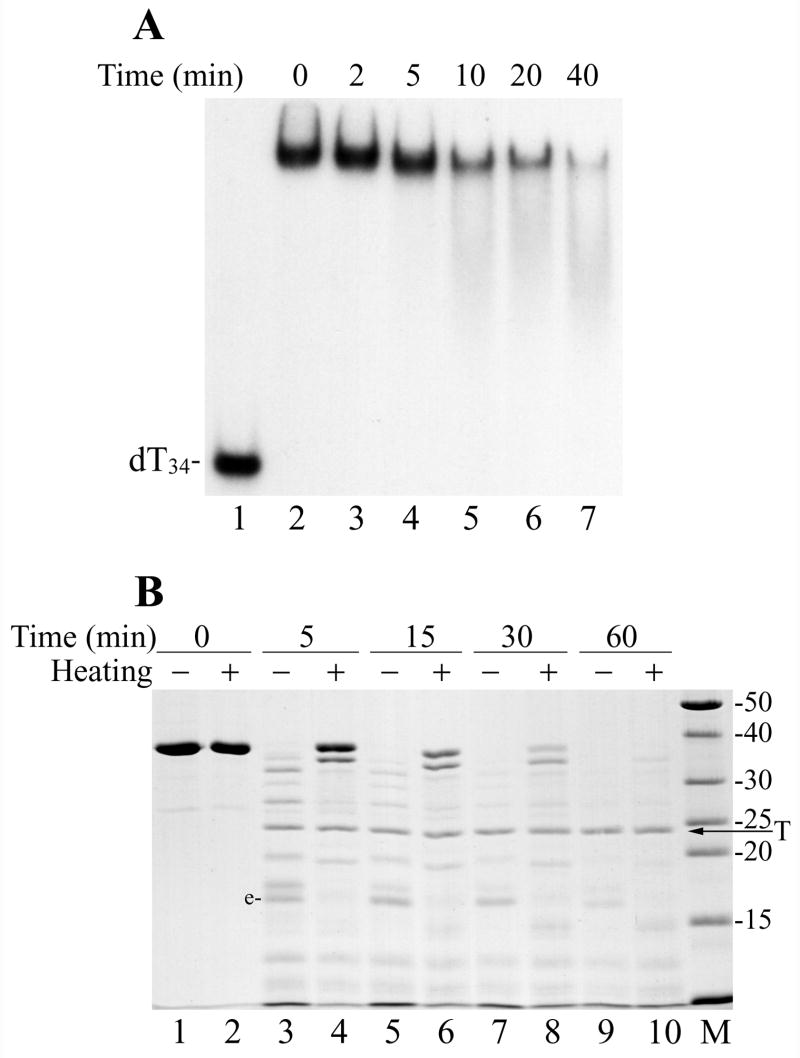
Figure 5.
Thermal inactivation of DBP binding to DNA and protein unfolding. (A) The his-tagged DBP (1.8 μM) in the storage buffer (50% glycerol, 10 mM Tris-HCl, pH 7.5, 0.2 M NaCl, 1 mM DTT) supplemented with 1% Nonidet P-40 was incubated at 50ºC as indicated above respective lanes and then was assayed for binding to 32P-labeled dT34 by EMSA. Lanes 1 and 2 show, respectively, controls without DBP and with non-heated DBP. (B) Trypsin digestion of the non-heated DBP and thermally unfolded DBP. Two 60-μl mixtures containing 3.6 μM DBP, 50 mM Tris-HCl, pH 7.5, 0.1 M NaCl, 12.5% glycerol, and 5 mM DTT were assembled on ice. The first mixture was left on ice, while the second was heated for 10 min at 70ºC. 10-μl portions were removed from both mixtures (lanes 1 and 2, respectively), then trypsin (18 μg/ml) was added and digestion was carried out at 30ºC. 10-μl portions were removed from the mixtures at 5 min (lanes 3 and 4), 15 min (lanes 5 and 6), 30 min (lanes 7 and 8), and 60 min (lanes 9 and 10). The reactions were terminated by the addition of 5.1 μl of loading buffer (3 mM PMSF, 190 mM Tris, pH 6.8, 4.3 M 2-mercaptoethanol, 6% SDS) followed by boiling for 3 min, and then analyzed by SDS-13% PAGE. The lanes with the non-heated and heated DBP are marked respectively by symbols “−“ and “+”. The arrow shows position of trypsin (T) in the gel.