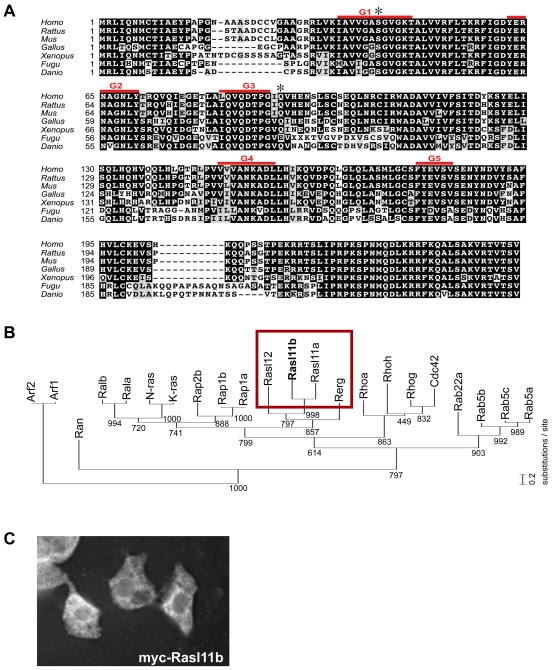
Figure 1. Rasl11b, an atypical cytoplasmic Ras small GTPase, is strongly conserved in Vertebrates.
(A) Zebrafish rasl11b encodes a Ras-related small GTPase of 244 amino acids (accession number DQ983377) containing the 5 highly conserved domains (G1–G5, overlined in red) responsible for the guanine nucleotide-dependent molecular switches. Rasl11b has no obvious orthologues in Drosophila melanogaster or Caenorhabditis elegans, but is highly conserved among vertebrates. Note that, in contrast to most of the Ras small GTPases, Rasl11b lacks a COOH-terminal CAAX motif and any known recognition signal for C-terminal lipidation found in Ras proteins such as farnesylation or palmitoylation allowing membrane anchorage. The amino acid positions mutated to create the activated forms Rasl11bS42V and Rasl11bQ82L are indicated with stars. (B) Phylogenic analysis of zebrafish small GTPase proteins. The degree of relatedness is indicated by the length of the vertical lines. Numbers indicate bootstrap support for nodes. Red box: Rasl11b, Rasl11a, Rasl12 and Rerg constitute an uncharacterized branch of Ras proteins devoid of lipid modification signals. (C) Epifluorescent microscopy of zebrafish embryonic cells expressing myc-Rasl11b revealed by immunostaining.