Fig. 4. Gel-shift analysis of rtAhR2s and rtARNTb interactions in vitro.
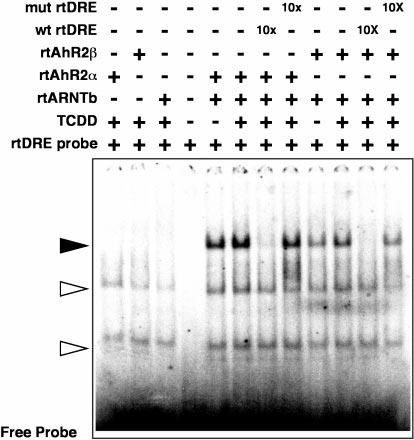
Equal amounts of in vitro translated rtAhR2α or rtAhR2β proteins were incubated with equal molar amounts of rtARNTb with, or without, 10 nm TCDD. The samples were then incubated with a 32P-labeled oligonucleotide probe derived from a DRE in the rainbow trout CYP1A enhancer. In some lanes a 10-fold molar excess of unlabeled competitor oligonucleotides was also added as indicated. The bound and free oligonucleotides were separated on a native acrylamide gel, and a phosphorimage of the dried gel is shown. The solid arrow indicates the rtAhR2·rtARNTb·DRE complexes. Open arrows indicate positions of nonspecific complexes.
