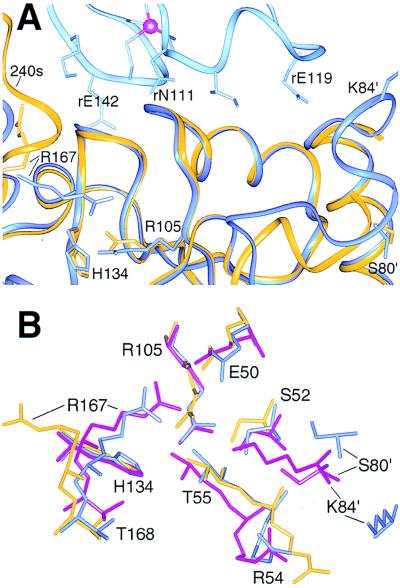
Figure 4.
Similar local conformations near the active sites of the isolated C trimer and the T state holoenzyme. Residues are labeled in the one-letter code. (A) Catalytic (dark blue) and regulatory (r, light blue) chains from the T state holoenzyme superimposed by the amino-terminal domain of the catalytic chains on chain Z (gold) of the C trimer. The 80s loop on the right is flexible in the isolated trimer, and the 240s loop on the left is ordered in chain Z by a crystal contact. (B) Superposition of the active site regions of chain Z (gold), a catalytic chain from the T state (blue), and PALA-liganded (magenta) holoenzyme structures. For clarity, PALA was omitted from the liganded structure. The ordered regions of the active sites in the isolated C trimer more closely resemble the T state holoenzyme. Diagnostic similarities are apparent for Thr-168 and the phosphate binding loop (Ser-52, Arg-54, and Thr-55). Because the isolated C trimer defines an active conformation, the distinct tertiary structure of the holoenzyme:PALA complex may result largely from ligand binding rather than the allosteric transition alone.