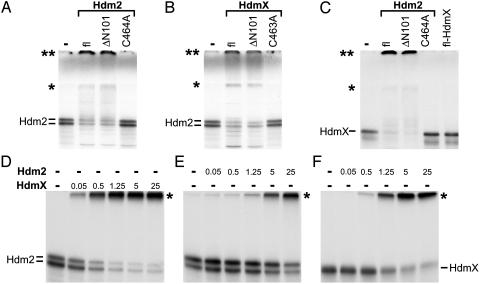
Fig. 3.
HdmX stimulates ubiquitination of Hdm2 and vice versa. In vitro-translated, radiolabeled Hdm2 (A and B) or HdmX (C) were incubated with various forms of bacterially expressed GST fusion proteins of Hdm2 (1 μg) and HdmX (1 μg) under standard in vitro ubiquitination conditions as indicated. fl, full-length. ΔN101 represents an Hdm2 or HdmX mutant, of which the N-terminal 101 aa of Hdm2 or HdmX were deleted; C464A and C463A, the respective cysteine residues of HdmX and Hdm2 were replaced by alanine. Note that, in this particular experiment, the ubiquitinated forms of both Hdm2 (A and B) and HdmX (C) run at two positions indicated by an asterisk and by a double asterisk. The position indicated by a single asterisk coincides with the top of the separating gel, and the position of the double asterisk coincides with the top of the stacking gel. The reason for the different migration behavior of the ubiquitinated forms (top of separating gel vs. top of stacking gel) is unknown, but note that analysis of the different forms by mass spectrometry revealed no differences with respect to the lysine residues of ubiquitin used for chain formation (see also legend to Fig. 4). (D–F) Titration analysis of HdmX-mediated ubiquitination of Hdm2 (D) and of Hdm2-mediated ubiquitination of Hdm2 (E) and HdmX (F). Amounts of Hdm2 and HdmX used are indicated in 10–1 μg. The running positions of ubiquitinated forms of Hdm2 and HdmX are indicated by an asterisk.