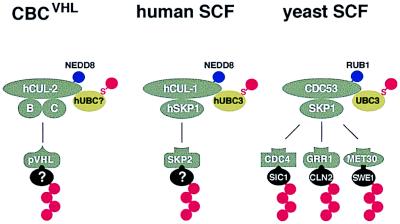
Figure 7.
Model of SCF-like protein complexes and their modification by NEDD8/RUB1. CBC and SCF complexes (gray) are structurally related. Yeast SCF ubiquitin ligase contains the cullin CDC53, SKP1, and one of several F-box proteins, which recruit specific substrates (black) to the complex leading to their ubiquitination (red chain). Ubiquitin conjugation via SCF is catalyzed by the UBC3CDC34 ubiquitin-conjugating enzyme (green), which forms a thioester-linked (S) intermediate with ubiquitin (red). Human cullin hCUL-1 is part of a similar SCF complex, whereas hCUL-2 associates with elongin B (B), the SKP1-related protein elongin C (C), and the pVHL tumor suppressor protein, which may function as a substrate-recruiting factor. Whether CBC can interact with alternative factors (other than pVHL) is not known. The cullins of all three complexes are modified by NEDD8 or RUB1 (blue).