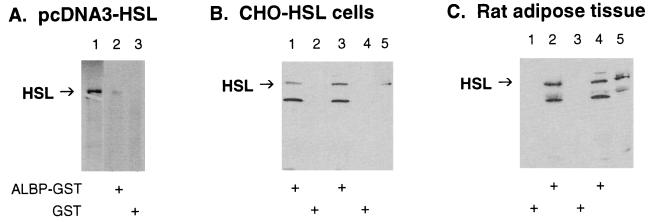
Figure 2.
Interaction of HSL with ALBP in vitro. (A) Interaction of ALBP–GST with in vitro-translated HSL. pcDNA3–HSL was in vitro translated with [35S]methionine by using the TNT transcription/translation system (lane 1). Extracts (400 μg) of E. coli transformed with pGEX–ALBP (ALBP–GST, lane 2) or with pGEX alone (GST, lane 3) were incubated with glutathione-agarose beads and with [35S]methionine-labeled HSL as described in Materials and Methods. Proteins that bound to the beads were eluted, separated on SDS/10% PAGE, and visualized by using a PhosphorImager. (B) Interaction of ALBP–GST with CHO cells overexpressing HSL. ALBP–GST (lanes 1 and 3) or GST (lanes 2 and 4) was incubated with glutathione beads as above. The beads were then incubated with extracts from CHO cells stably overexpressing HSL. Proteins that bound to the beads were eluted, separated on SDS/10% PAGE, immunoblotted with anti-rat HSL fusion protein antibodies, and visualized by using enhanced chemiluminescence. Lane 5 contains extracts (25 μg) of CHO cells overexpressing HSL that were used in the incubations. (C) Interaction of ALBP–GST with extracts of rat adipose tissue. ALBP–GST (lanes 2 and 4) or GST (lanes 1 and 3) was incubated with glutathione beads as above. The beads were then incubated with extracts prepared from rat epididymal fat pads. Proteins that bound to the beads were eluted, separated on SDS/10% PAGE, immunoblotted with anti-rat HSL fusion protein antibodies, and visualized by using enhanced chemiluminescence. Lane 5 contains extracts (25 μg) of adipose tissue homogenate that were used in the incubations.