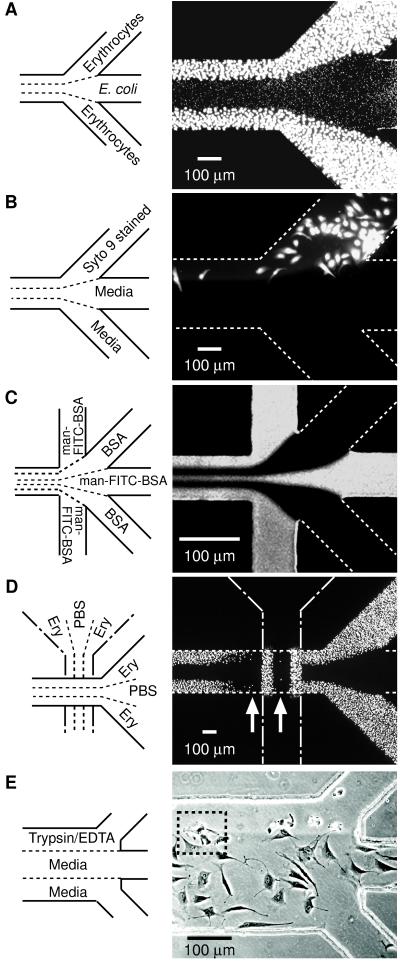
Figure 2.
Examples of types of patterns that can be formed by laminar flow patterning. (A) Two different cell types patterned next to each other. A suspension of chicken erythrocytes was placed in inlets 1 and 3, and PBS in inlet 2 and allowed to flow by gravitational force for 5 min followed by a 3-min PBS wash; this flow formed the pattern of bigger cells (outer lanes). Next, a suspension of E. coli (RB 128) was placed in inlet 2 and PBS in inlets 1 and 3 and allowed to flow by gravitational forces for 10 min followed by a 3-min PBS wash; this flow created the pattern of smaller cells (middle lane). Both cell types adhered to the Petri dish by nonspecific adsorption. Cells were visualized with Syto 9 (15 μM in PBS). (B) Pattern of selectively stained BCE cells. BCE cells suspended in chemically defined medium (≈106 cells per ml) were introduced into the capillary network (pretreated with fibronectin) and incubated in 10% CO2 at 37 °C for 4 hr. After removing nonadherent cells by washing with media (1% wt/vol BSA/DMEM), Syto 9 (15 μM in BSA/DMEM) and media were allowed to flow from the designated inlets for 5 min under gentle aspiration, and the system was washed for 3 min with media. (C) Pattern obtained at a junction where five inlets converge into a 75-μm channel (see caption Fig. 1C for procedures). (D) Crisscross patterning of chicken erythrocytes. Erythrocytes were patterned initially in the vertical direction (procedure same as A). The PDMS membrane used in this initial patterning was demounted and a different PDMS membrane placed in a direction 90° rotated from the first pattern (that is, horizontal). The capillary system created by the second PDMS membrane was used to pattern erythrocytes in the horizontal (right-to-left) direction. The resulting pattern of erythrocytes in the second capillary system was visualized by Syto 9 (15 μM in PBS). (E) Patterned detachment of BCE cells by treatment with trypsin/EDTA. Cells were allowed to adhere and spread in a fibronectin-treated capillary network for 6 hr and nonadherent cells removed by washing. Trypsin/EDTA and media were allowed to flow from the designated inlets for 12 min by gravity. A–D are fluorescence micrographs taken from the top through PDMS. E is a phase-contrast image observed by an inverted microscope looking through the polystyrene Petri dish. White dotted lines identify channels not visible with fluorescence microscopy.