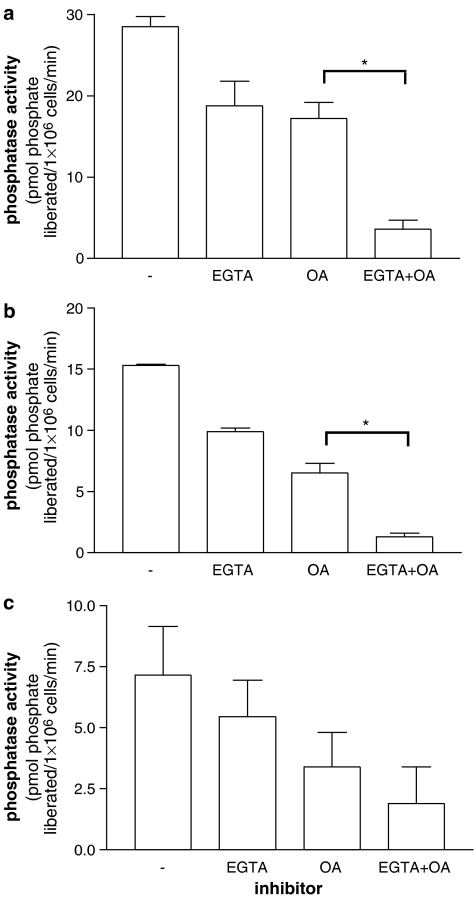
Figure 6.
Ca2+-dependent protein phosphatase activities in (a) mast cells, (b) mononuclear cells and (c) basophils. Dephosphorylation of radiolabelled RII phosphopeptide was followed over 5 min in the presence of Ca2+ (1 mM) and in the absence (−) or presence of EGTA (5 mM) and/or okadaic acid (OA, 50 nM). Protein phosphatase activity is expressed as the liberation of phosphate from RII peptide per minute per 106 cells. Purities were 95±1% (range 92–99%) for mast cells and 97±2% (range 91–99%) for basophils. EGTA reduced the protein phosphatase activity to a statistically significant (*P<0.05) degree in mast cells and mononuclear cells but not in basophils (comparison between protein phosphatase activities in the presence of OA and EGTA+OA). Results are means and vertical lines, s.e.m., n=4. Note that the y axis range of values differs for each panel.