Abstract
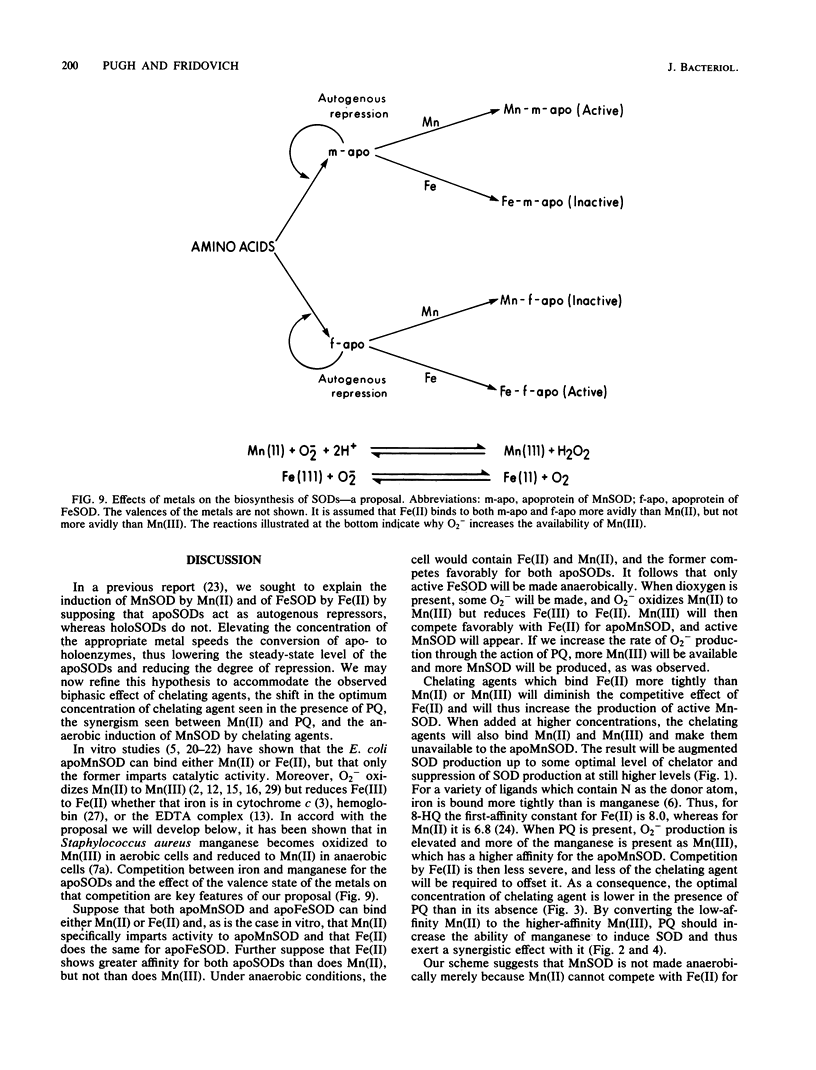
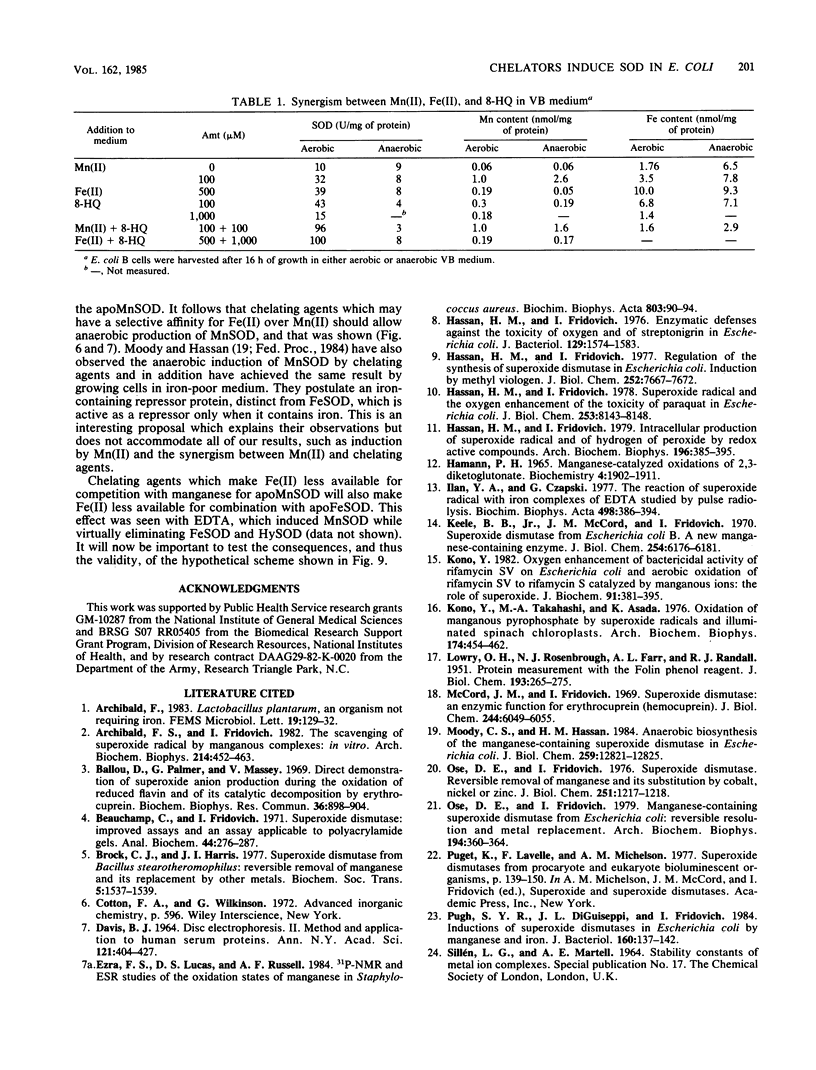
The effects of metal salts, chelating agents, and paraquat on the superoxide dismutases (SODs) of Escherichia coli B were explored. Mn(II) increased manganese-containing SOD (MnSOD), whereas Fe(II) increased iron-containing SOD (FeSOD). Chelating agents induced MnSOD but decreased FeSOD and markedly increased the degree of induction seen with Mn(II). Paraquat also exerted a synergistic effect with Mn(II). High levels of MnSOD were achieved in the combined presence of Mn(II), chelating agent, and paraquat. All of these effects were dependent on the presence of oxygen. MnSOD, not ordinarily present in anaerobically grown E. coli cells, was present when the cells were grown anaerobically in the presence of chelating agents. These results are accommodated by a scheme which incorporates autogenous repression by the apoSODs and competition between Fe(II) and Mn(II) for the metal-binding sites of the apoSODs. It is further supposed that oxygenation and intracellular O2- production favor MnSOD production because O2- oxidizes Mn(II) to Mn(III), which competes favorably with Fe(II) for the apoSODs.
Full text
PDF
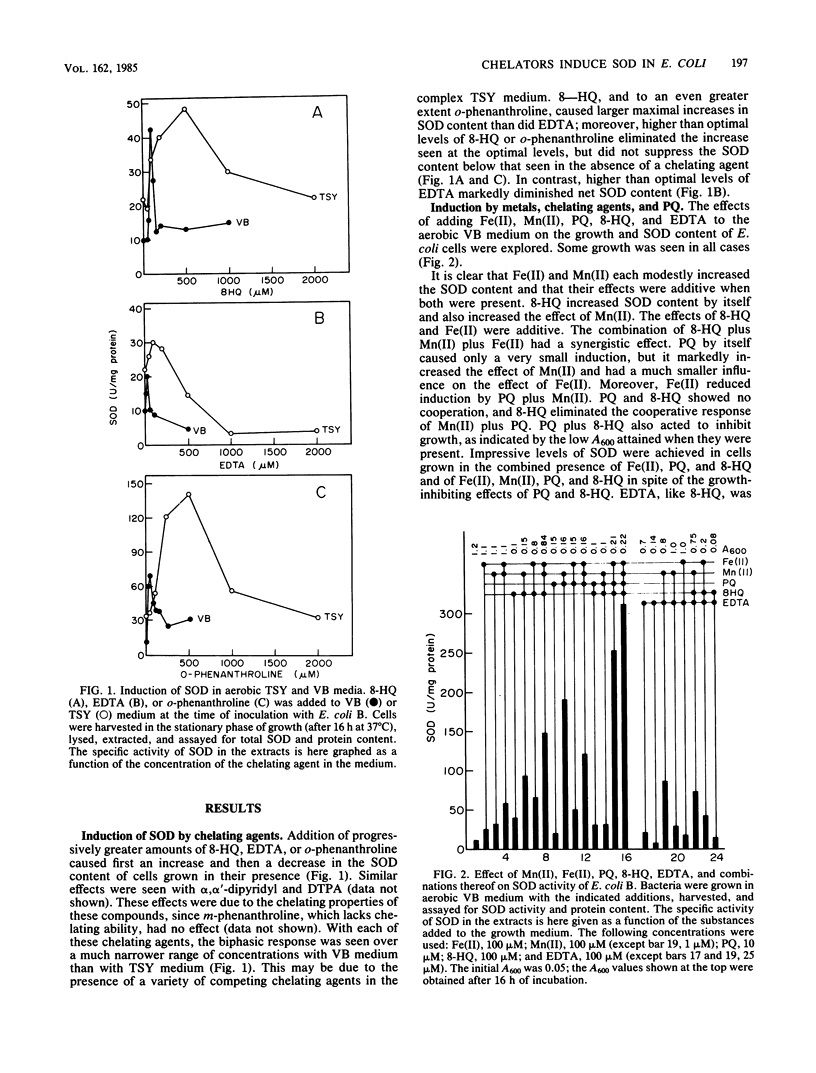
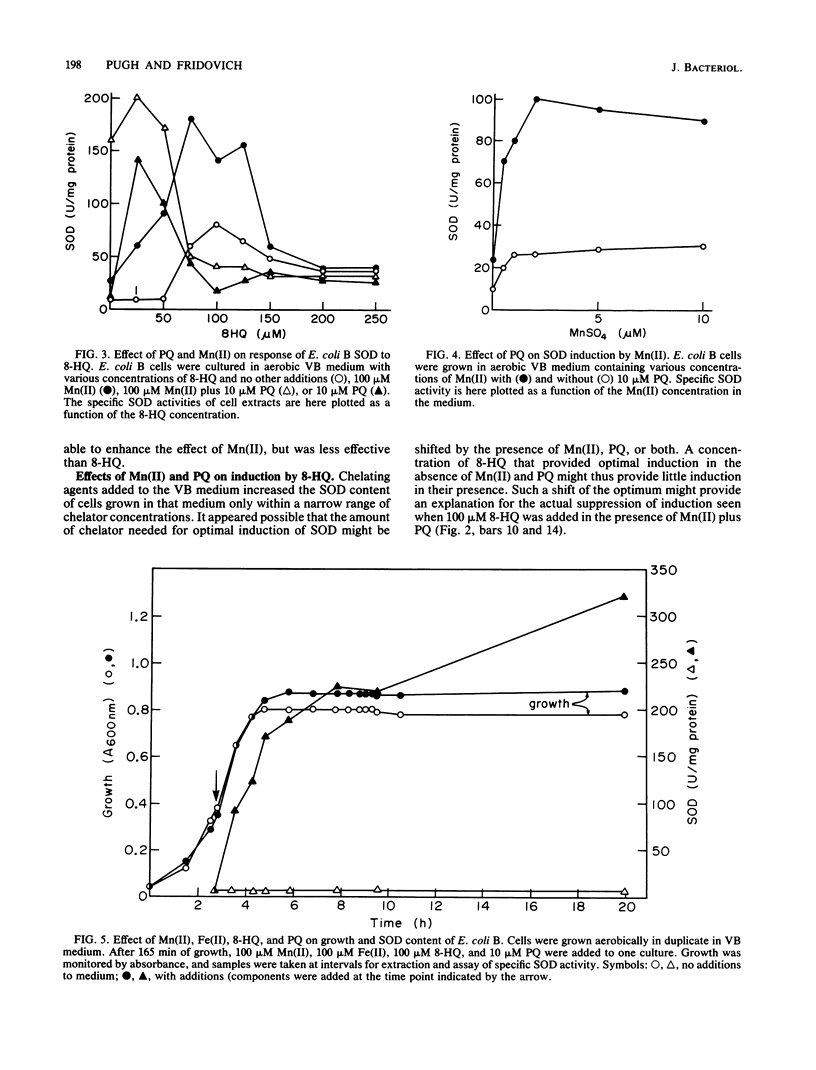
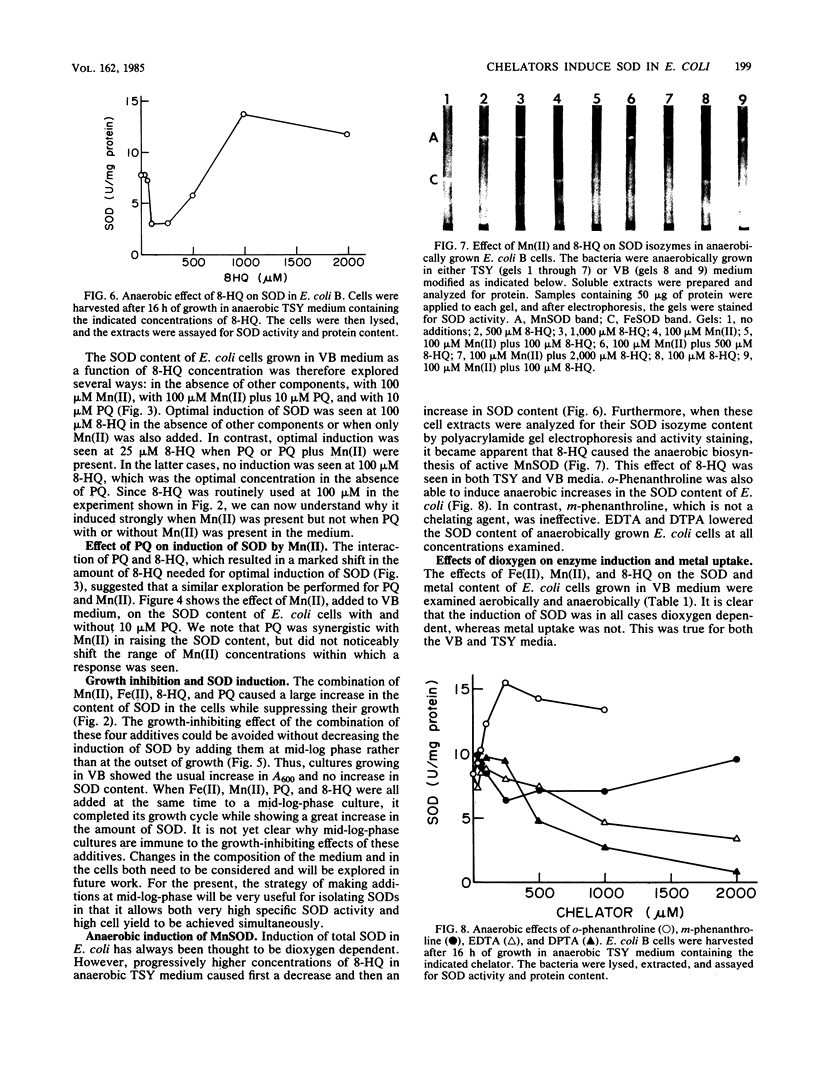

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald F. S., Fridovich I. The scavenging of superoxide radical by manganous complexes: in vitro. Arch Biochem Biophys. 1982 Apr 1;214(2):452–463. doi: 10.1016/0003-9861(82)90049-2. [DOI] [PubMed] [Google Scholar]
- Ballou D., Palmer G., Massey V. Direct demonstration of superoxide anion production during the oxidation of reduced flavin and of its catalytic decomposition by erythrocuprein. Biochem Biophys Res Commun. 1969 Sep 10;36(6):898–904. doi: 10.1016/0006-291x(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Brock C. J., Harris J. I. Superoxide dismutase from Bacillus stearothermophilus: reversible removal of manganese and its replacement by other metals [proceedings]. Biochem Soc Trans. 1977;5(5):1537–1539. doi: 10.1042/bst0051537. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Ezra F. S., Lucas D. S., Russell A. F. 31P-NMR and ESR studies of the oxidation states of manganese in Staphylococcus aureus. Biochim Biophys Acta. 1984 Feb 17;803(1-2):90–94. doi: 10.1016/0167-4889(84)90059-4. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Enzymatic defenses against the toxicity of oxygen and of streptonigrin in Escherichia coli. J Bacteriol. 1977 Mar;129(3):1574–1583. doi: 10.1128/jb.129.3.1574-1583.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch Biochem Biophys. 1979 Sep;196(2):385–395. doi: 10.1016/0003-9861(79)90289-3. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Regulation of the synthesis of superoxide dismutase in Escherichia coli. Induction by methyl viologen. J Biol Chem. 1977 Nov 10;252(21):7667–7672. [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Superoxide radical and the oxygen enhancement of the toxicity of paraquat in Escherichia coli. J Biol Chem. 1978 Nov 25;253(22):8143–8148. [PubMed] [Google Scholar]
- Ilan Y. A., Czapski G. The reaction of superoxide radical with iron complexes of EDTA studied by pulse radiolysis. Biochim Biophys Acta. 1977 Jul 21;498(1):386–394. doi: 10.1016/0304-4165(77)90277-x. [DOI] [PubMed] [Google Scholar]
- Keele B. B., Jr, McCord J. M., Fridovich I. Superoxide dismutase from escherichia coli B. A new manganese-containing enzyme. J Biol Chem. 1970 Nov 25;245(22):6176–6181. [PubMed] [Google Scholar]
- Kono Y. Oxygen Enhancement of bactericidal activity of rifamycin SV on Escherichia coli and aerobic oxidation of rifamycin SV to rifamycin S catalyzed by manganous ions: the role of superoxide. J Biochem. 1982 Jan;91(1):381–395. doi: 10.1093/oxfordjournals.jbchem.a133698. [DOI] [PubMed] [Google Scholar]
- Kono Y., Takahashi M. A., Asada K. Oxidation of manganous pyrophosphate by superoxide radicals and illuminated spinach chloroplasts. Arch Biochem Biophys. 1976 Jun;174(2):454–462. doi: 10.1016/0003-9861(76)90373-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Moody C. S., Hassan H. M. Anaerobic biosynthesis of the manganese-containing superoxide dismutase in Escherichia coli. J Biol Chem. 1984 Oct 25;259(20):12821–12825. [PubMed] [Google Scholar]
- Ose D. E., Fridovich I. Manganese-containing superoxide dismutase from Escherichia coli: reversible resolution and metal replacements. Arch Biochem Biophys. 1979 May;194(2):360–364. doi: 10.1016/0003-9861(79)90628-3. [DOI] [PubMed] [Google Scholar]
- Ose D. E., Fridovich I. Superoxide dismutase. Reversible removal of manganese and its substitution by cobalt, nickel or zinc. J Biol Chem. 1976 Feb 25;251(4):1217–1218. [PubMed] [Google Scholar]
- Pugh S. Y., DiGuiseppi J. L., Fridovich I. Induction of superoxide dismutases in Escherichia coli by manganese and iron. J Bacteriol. 1984 Oct;160(1):137–142. doi: 10.1128/jb.160.1.137-142.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slykhouse T. O., Fee J. A. Physical and chemical studies on bacterial superoxide dismutases. Purification and some anion binding properties of the iron-containing protein of Escherichia coli B. J Biol Chem. 1976 Sep 25;251(18):5472–5477. [PubMed] [Google Scholar]
- Stallings W. C., Pattridge K. A., Strong R. K., Ludwig M. L. Manganese and iron superoxide dismutases are structural homologs. J Biol Chem. 1984 Sep 10;259(17):10695–10699. [PubMed] [Google Scholar]
- Steinman H. M. The amino acid sequence of mangano superoxide dismutase from Escherichia coli B. J Biol Chem. 1978 Dec 25;253(24):8708–8720. [PubMed] [Google Scholar]
- Sutton H. C., Roberts P. B., Winterbourn C. C. The rate of reaction of superoxide radical ion with oxyhaemoglobin and methaemoglobin. Biochem J. 1976 Jun 1;155(3):503–510. doi: 10.1042/bj1550503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Yost F. J., Jr, Fridovich I. An iron-containing superoxide dismutase from Escherichia coli. J Biol Chem. 1973 Jul 25;248(14):4905–4908. [PubMed] [Google Scholar]