Figure 1.
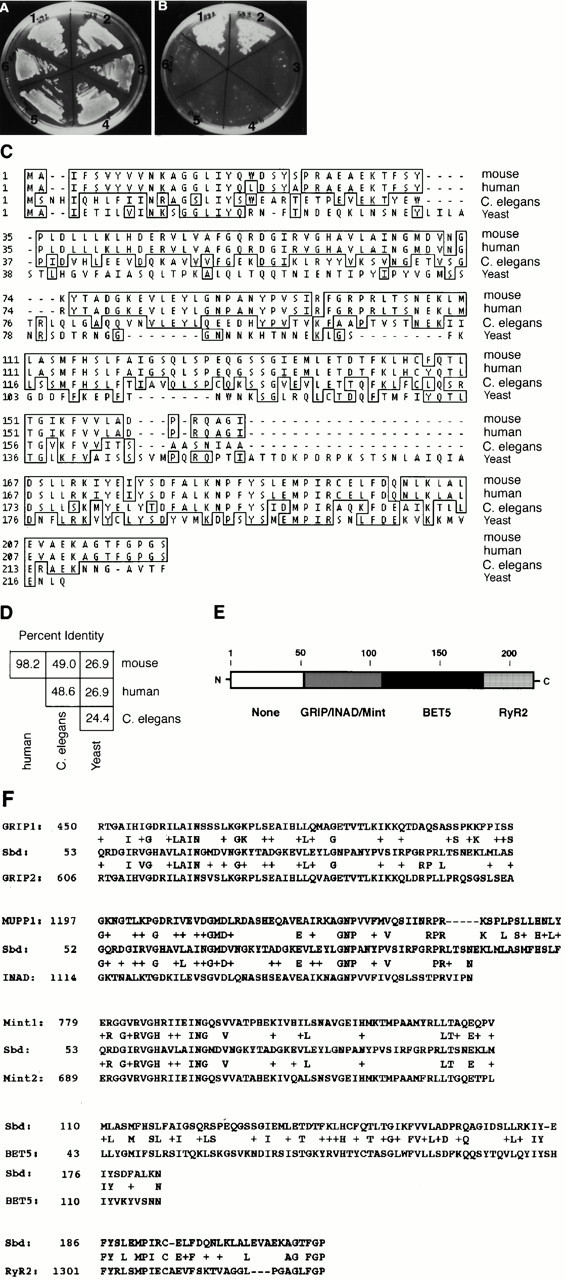
Synbindin is a novel protein that interacts with the EFYA motif of syndecans. (A and B) Binding of clone 28 (synbindin) to the syndecan cytoplasmic domains. Two-hybrid assays were performed with the Leu2 activation domain containing clone 28 and the LexA DNA–binding domain containing one of following syndecan cytoplasmic domains: syndecan-2 (1); syndecan-4 (2); a scrambled amino acid sequence of syndecan-2 (3); syndecan-4ΔEFYA (4); and syndecan-2ΔEFYA (5 and 6). Transformants were grown on a Leu−/Trp− plate (A) or a His−/Leu−/Trp− plate (B). (C) Amino acid sequences of mouse synbindin deduced from its full-length cDNA sequence in comparison with the putative human synbindin (AAF21897.1), C. elegans F36D4.2 (AAA93486), and the yeast p23 (Sacher et al. 1998). Identical amino acid residues are shown in a box. The nucleotide sequence data of mouse synbindin is available from GenBank/EMBL/DDBJ under accession number AF233340. (D) Percent amino acid identity between mouse synbindin and its human, nematode, and yeast homologues. (E) A model of the synbindin molecule. Each box represents a region that shows homology with the known protein(s) indicated below the box. Numbers indicate amino acid residues. (F) Alignment of mouse synbindin (Sbd) with human GRIP1 (AJ133439) and rat GRIP2 (AF112182), human MUPP1 (AF093419) and human INAD (AJ001306), rat Mint1 (AF029105) and human Mint2 (AF029108), yeast BET5 (NP_013634), and human RyR-2 (X98330).
