Abstract
Orphan G-protein-coupled receptors that have recently been paired with their cognate ligand are an often untapped resource for novel drug development. The KISS1 receptor (previously designated GPR54) has been paired with biologically active cleavage peptides of the KiSS-1 gene product, the kisspeptins (KP). The focus of this review is the emerging pharmacology and physiology of the KP. Genetic linkage analysis in humans revealed that mutations in KISS1 (GPR54, AXOR12 or hOT7T175) result in idiopathic hypogonadotrophic hypogonadism and knockout mouse studies confirmed this finding. Identification of KISS1 (GPR54) as a molecular switch for puberty subsequently led to the discovery that KP activate the GnRH cascade. Prior to the role of KISS1 (GPR54) in puberty being described, KP had been shown to be inhibitors of tumour metastasis across a range of cancers. Subsequently the mechanism of this inhibition has been suggested to be via altered cell motility and adhesiveness. PCR detected highest expression of KP and KISS1 (GPR54) in placenta, and changes in KP levels throughout pregnancy and expression in trophoblasts suggests a role in placentation. Placentation and metastasis are invasive processes that require angiogenesis. Investigation of KISS1 (GPR54) and KP in vasculature revealed discrete localisation of KISS1 (GPR54) to blood vessels prone to atherosclerosis and a potent vasoconstrictor action. A role for KP has also been shown in whole body homeostasis. KP are multifunctional peptides and further investigation is required to fully elucidate the complex pathways regulated by these peptides and how these pathways integrate in the whole body system.
Keywords: orphan G-protein-coupled receptor, kisspeptin, KISS1, KiSS-1, GPR54, puberty, metastasis, placentation, cardiovascular, vasoconstrictor
Introduction
Sequencing of the human genome is now 99% complete (International Human Genome Sequencing Consortium, 2004). A major challenge that has arisen from this new information is to translate genomic sequences, as efficiently as possible, into novel therapeutic targets. A significant proportion of genes within the human genome, although disease related, do not represent targets for drug development, as they lack the ability to bind and respond to small molecule agonists/antagonists. Ligand binding at the Class 1 G-protein-coupled receptors (GPCRs) often involves only a small number of residues within the binding pocket, facilitating the binding of small molecules. As a result GPCRs are the molecular target of up to 50% of marketed drugs in clinical use (Wise et al., 2002).
Bioinformatics has been used to identify the coding sequence of all GPCRs in the human genome, and the International Union of Pharmacology (IUPHAR) has compiled a comprehensive list of all non-sensory GPCRs. Currently this includes 367 gene sequences, with 238 being known receptors with characterized endogenous ligands and 129 genes encoding novel sequences for which the cognate ligand is not yet known, of these 86 share sequence homology with the Class 1 receptors (Foord et al., 2005; Maguire and Davenport, 2005). In the last 10 years more than 50 ‘orphan' GPCRs have been paired with their cognate ligands using the reverse pharmacology approach. In 2001, one of the orphans KISS1 (previously designated GPR54, AXOR12, hOT7T175) was paired with three biologically active cleavage peptides of the kisspeptin gene (KiSS-1) gene product isolated from human placenta, the kisspeptins (KP)-54, KP-13 and KP-10 (Kotani et al., 2001; Muir et al., 2001; Ohtaki et al., 2001). In addition to previously described roles in cancer metastasis and placentation, the KISS1 (GPR54) receptor system has received growing attention following the discovery that it acts as an unexpected molecular switch for puberty. Therefore, understanding the complex interactions of the KP and the KISS1 (GPR54) receptor in these physiological and pathophysiological processes may be critical in development of novel therapeutic agents targeting this receptor. The present review summarizes published data on the physiology and pharmacology of this emerging receptor system.
Kisspeptin nomenclature
Throughout this review the receptor gene name will be given as KISS1 receptor gene (KISS1R) and peptide gene name as KiSS-1 according to the Human Genome Organisation nomenclature. Receptor protein name will be given as KISS1, according to standard IUPHAR nomenclature (Davenport and Mead, 2005). For clarity, the orphan receptor nomenclature GPR54 will additionally be given in brackets wherever referring to the KISS1 receptor. The kisspeptins, as a collective group, will be abbreviated as KP (Table 1). Where individual kisspeptins are referred to, their amino acid sequence length will also be given, KP-54 (previously designated metastin), KP-13 and KP-10.
Table 1.
KISS1 (GPR54) receptor and KP nomenclature, Swiss-Prot accession number and chromosomal location in human, rat and mouse
| Nomenclature | Species | Accession number | Chromosome location | Gene name |
|---|---|---|---|---|
| KISS1 (GPR54) (receptor) | Human | Q969F8 | 19p13.3 | KISS1R |
| KISS1 (GPR54) (receptor) | Rat | Q924U1 | 7q11 | KISS1R |
| KISS1 (GPR54) (receptor) | Mouse | Q91V45 | 10 C1 | KISS1R |
| KP (peptide) | Human | Q15726 | 1q32.1 | KiSS-1 |
| KP (peptide) | Rat | Q7TSB7 | 13q13 | KiSS-1 |
| KP (peptide) | Mouse | Q6Y4S4 | 1 E4 | KiSS-1 |
Abbreviation: KP, kisspeptin.
Discovery of the kisspeptins
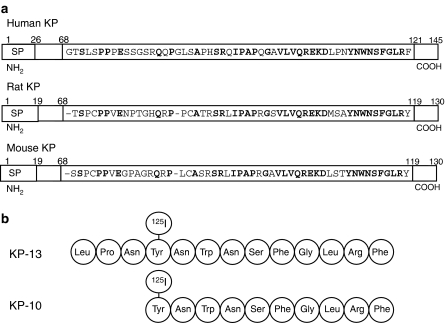
The kisspeptins (KP) were originally identified in 1996 from a metastasis suppressor gene, KiSS-1, in malignant melanomas (Lee et al., 1996). Initially, the largest cleavage product, KP-54, was identified for its ability to suppress metastatic potential in human melanoma cells. Its expression also resulted in suppression of melanoma metastasis in athymic nude mice and it was therefore termed metastin. Three biologically active cleavage peptides of the KiSS-1 gene product have been isolated from human placenta, KP-54, KP-13 and KP-10 (Figure 1a) (Kotani et al., 2001; Ohtaki et al., 2001) and are called kisspeptins. This term originated from the cDNA designation KiSS-1, which was derived from a combination of interim laboratory nomenclature for putative suppressor sequences and acknowledgement of the laboratories location in Hershy, USA (Smith et al., 2005b; Lee et al., 1996). The full-length KP protein (KP-145) (Figure 1) has a PEST sequence (proline, glutamic acid, serine, threonine and aspartic acid residue-rich sequence), which although upstream of the KP-13 and KP-10 sequence, is still present in the KP-54 sequence. This motif predisposes proteins for ubiquitination and proteosome degradation and suggests that cytosolic KP-145 would have a short half-life (Harms et al., 2003). Presence of a putative signal peptide sequence predicts that the KP may be secreted peptides (Yan et al., 2001). In rat and mouse, the longest KP cleavage fragment is composed of 52 amino acids. Although overall homology of human KiSS-1 gene products to rat and mouse is relatively low (∼52%), KP-10 is highly conserved between human, mouse and rat, with only one amino acid difference in the sequence between species. Initial molecular localization has revealed limited expression in both the periphery and the brain, with particularly high expression in the placenta, although variation in reported expression exists (Lee et al., 1996; Muir et al., 2001; Ohtaki et al., 2001).
Figure 1.
(a) The structure of the KP pre-pro-protein KP-145 in human, rat and mouse. Amino acid sequence of human KP-54 and rat and mouse KP-52 is given. Amino acids in bold are conserved cross-species. (b) [125I]KP-13 has been used to pharmacologically characterize KISS1 (GPR54) in native human tissue (Mead et al., 2006) and [125I]KP-10 in cell lines artificially expressing the receptor (Kotani et al., 2001). SP, signal peptide.
Pairing of the KP with KISS1 (GPR54)
The novel receptor KISS1 (previously designated GPR54, AXOR12 or hOT7T175) was isolated in 1999 by a degenerate PCR search of rat brain (Lee et al., 1999). It shares significant homology with galanin receptors (∼44–45%) and basic local alignment search tool searching revealed the human orthologue (Table 1). However, no specific binding of 125I-human galanin was observed in cells transfected with the KISS1 (GPR54) receptor. The human receptor gene contains four introns and shares a translated amino acid identity of ∼81% with the rat and ∼85% with mouse, increasing to 100% identity in the TM regions. In 2001, KISS1 (GPR54) was paired with the KP by three different groups (Kotani et al., 2001; Muir et al., 2001; Ohtaki et al., 2001). Additionally, a fourth group identified less potent activation of KISS1 (GPR54) by FMRFamides, including derivatives of antho-RWamide I (Clements et al., 2001). In cell lines, artificially expressing KISS1 (GPR54) radioligand binding using [125I]KP-10 revealed a single high affinity binding site (Kotani et al., 2001). For the native receptor, novel radioligand [125I]KP-13 has been used to define the pharmacological criteria of saturable, specific and reversible binding in human cardiovascular tissues with density of binding (Bmax) of 7.65±0.95 fmol mg−1 protein in aorta smooth muscle (Mead et al., 2006) (Figure 1b). In agreement with KP expression, KISS1 (GPR54) mRNA has been found most abundantly in placenta (Clements et al., 2001; Kotani et al., 2001; Muir et al., 2001; Ohtaki et al., 2001).
Downstream signalling of KISS1 (GPR54) activation
Activation of KISS1 (GPR54) results in intracellular calcium mobilization that is not affected by pertussis toxin and does not result in changes in cAMP accumulation, suggesting that it is a Gq-coupled receptor (Kotani et al., 2001; Muir et al., 2001). Numerous studies have sought to further elucidate the downstream signalling pathways activated via stimulation of KISS1 (GPR54) by KP. However, precise mechanisms remain controversial. This potentially reflects a complex network of signalling, resulting in diverse physiological responses (Harms et al., 2003). At the top of this cascade, KP activation of KISS1 (GPR54) has been shown to simultaneously result in release of arachidonic acid (Kotani et al., 2001) and stimulation of the mitogen-activated protein kinase (MAPKs) extracellular signal-regulated kinase (ERK)1 and ERK2 (Kotani et al., 2001; Yan et al., 2001; Ringel et al., 2002; Masui et al., 2004; Mullins and Mullins, 2004; Becker et al., 2005). This has been attributed to increased phosphorylation of MAPK. However, while some studies found no activation of p38 or pAkt (Ringel et al., 2002; Goldberg et al., 2003), others found activation of both signalling pathways (Masui et al., 2004; Mullins and Mullins, 2004). Additionally, other kinases are reported to be activated by KISS1 (GPR54), including p42/44, PKC, PLC, myeloid cell leukaemia 1, calcium/calmodulin-dependent kinases and tyrosine kinases (Mullins and Mullins, 2004; Becker et al., 2005). Microarray platforms enable screening of thousands of genes in one experiment by attaching DNA molecules to a microchip and using this to probe a sample of interest to look for changes in gene expression. Microarray analysis of KISS1 (GPR54) regulated genes detected upregulation of myocyte-enriched calcineurin interacting protein, a known inhibitor of the vascular endothelial growth factor-regulated protein, calcineurin. No activation of the stress-activated protein kinase/c-jun N-terminal kinase has yet been identified (Kotani et al., 2001; Masui et al., 2004).
KP and matrix metalloproteinases
Downregulation of one or both of the gelatinase matrix metalloproteinases (MMPs), MMP-2 and MMP-9, by KP (Yan et al., 2001; Bilban et al., 2004; Hesling et al., 2004; Qiao et al., 2005) has been shown. KP have been described as regulators of MMPs at both the transcriptional and protein level. Transcriptional changes have been shown not to function through the MAPK signalling pathways. Instead, nuclear factor-κB binding to the MMP-9 promoter region, necessary for expression of MMP-9, was reduced (Yan et al., 2001). At the protein level, interaction between the N-terminal 48 amino acids of KP-145 and pro-MMP-2 or pro-MMP-9 has been shown to form a stable complex, although the physiological consequences of this interaction have not been investigated. Significantly, active MMPs can cleave the Glycine118-Leucine119 bond of KP, resulting in the removal of the C terminal three amino acids, leading to inactivation of KP. This may represent a regulatory feedback mechanism between KP and MMPs (Takino et al., 2003).
KP and cancer metastasis
One strategy for identifying genes involved in metastasis is to inject genes of interest into highly metastatic cells lines and observe changes in their ability to metastasize when injected into athymic nude mice. Transfer of normal human chromosome 6 into metastatic malignant melanoma cell lines C8161 and MelJuSo suppressed metastasis by 95% in this model, without affecting tumourigenicity or local invasiveness (Miele et al., 1996; Lee and Welch, 1997). Identification of the genes responsible for this phenotype on chromosome 6 was attempted using a modified subtractive hybridization method. This resulted in a number of candidate genes being identified. However only one gene, KiSS-1, was expressed in non-metastatic cells and was absent from the metastatic parental line (Miele et al., 1996). This finding was functionally confirmed by construction of a KiSS-1 vector, transfected into C8161 and injected into athymic nude mice. Metastatic ability, when compared to injection of C8161 alone, was reduced from an average of 50 metastases to only 1 (Miele et al., 1996). KiSS-1 maps to chromosome 1, suggesting that the element causing inhibition of metastasis on chromosome 6 may be an important regulator of the KP.
Upstream regulators of KP mediated inhibition of metastasis
Following the identification of a regulatory role for chromosome 6 on KiSS-1, research has sought to determine the exact gene responsible. Within region 6q16.3–q23, a number of candidate genes including kinases, vitamin D receptor interacting proteins and arginase-1 were identified (Harms et al., 2003). Microarray comparing genes in this region of metastatic and non-metastatic tumours identified the candidate gene CRSP3 that inhibited metastasis, but cells remained tumourigenic, as with the KP. CRSP3 is a part of the vitamin D receptor-related co-activator complexes and could therefore regulate KiSS-1 via a multitude of mediating factors (Goldberg et al., 2003). Additionally, direct interaction of KiSS-1 has been identified with two transcription factors, activator protein-2α and specificity protein-1, both of which have been shown to be important regulators of genes involved in tumourigenesis, metastasis and development (Mitchell et al., 2006).
Mechanistic insights into KP and cancer
In cell motility assays, which assessed the ability of cells to move towards foetal calf serum (FCS), KP-54 inhibited chemotaxis (Ohtaki et al., 2001; Masui et al., 2004). Furthermore, in an invasion assay testing migration through a Matrigel-coated filter, KP-54 additionally inhibited FCS-induced cell invasion (Ohtaki et al., 2001), an effect possibly mediated by downregulation of MMPs by KP (Hesling et al., 2004). Changes in the cell cytoskeleton can induce an adhesive cell type. In an assay to monitor actin microfilament reorganization, KP-10 stimulated the formation of stress fibres, a process abolished by pre-treatment with C3 exo-enzyme suggesting the activation of Rho G-proteins in the downstream pathway (Kotani et al., 2001). Focal adhesion formation was also induced by KP-54, with increased phosphorylation of focal adhesion kinase and paxillin, which are essential for formation of focal adhesions (Ohtaki et al., 2001). However, a separate study showed that in a cellular adhesion assay, KP-54 did not inhibit cellular adhesion, although it did inhibit motility and stimulated changes in cell morphology by transformation of actin filaments (Hori et al., 2001). Therefore, it remains to be determined if KP inhibit cell invasion by altered cell motility, altered adhesiveness, or a combination of both.
Induction of apoptosis, as a mechanism for metastasis inhibition by KP, was not identified (Kotani et al., 2001), although recently (Becker et al., 2005) have used Microarray analysis in the human mammary carcinoma cell line MDA-MB-435S to identify upregulation of a number of genes involved in cell cycle control and apoptosis by KP.
Clinical evidence for a role in cancer
In order to confirm importance of the KP, not solely as inhibitors of metastasis in malignant melanoma but as regulators of metastatic potential in a variety of cancers, changes were detected in KP and KISS1 (GPR54) in native cancer cells from metastatic and non-metastatic tumours (Table 2). The majority of these report decreased expression of KP in primary and metastatic tumours, with some reporting a complete absence of KP in metastases (Shirasaki et al., 2001; Sanchez-Carbayo et al., 2003b). KISS1 (GPR54) expression levels have not been reported to change in endometrial and pancreatic cancer; however, this is not a consistent finding with other reports of increases or decreases in expression.
Table 2.
Studies comparing expression of KP/KISS1 (GPR54) in metastatic and non-metastatic tumours across a variety of cancer types
| Cancer type | Changes in KP/KISS1 (GPR54) in metastatic tumours | Reference |
|---|---|---|
| Bladder cancer | Decreased KP | Sanchez-Carbayo et al. (2003b) |
| Breast carcinoma | Decreased KP | Lee and Welch (1997); Mitchell et al. (2006) |
| Choriocarcinoma | Decreased KP and KISS1 (GPR54) | Janneau et al. (2002) |
| Colon cancer | Not measured | Wisotzkey et al. (1997) |
| Endometrial carcinoma | Decreased KP, no change in KISS1 (GPR54) | Jiang et al. (2005) |
| Oesophageal carcinoma | Decreased KP and KISS1 (GPR54) | Ikeguchi et al. (2004) |
| Gastric carcinoma | Decreased KP | Dhar et al. (2004) |
| Hepatocellular carcinoma | Increased KP and KISS1 (GPR54) | Ikeguchi et al. (2003) |
| Malignant melanoma | Decreased KP | Lee et al. (1996); Shirasaki et al. (2001); Hesling et al. (2004) |
| Osteosarcoma | Decreased KP | Sanchez-Carbayo et al. (2003a) |
| Ovarian cancer | Decreased KP | Ohtaki et al. (2001) |
| Pancreatic cancer | Decreased KP, no change in KISS1 (GPR54) | Masui et al. (2004) |
| Papillary thyroid cancer | Decreased KP, increased KISS1 (GPR54) | Ringel et al. (2002) |
Correlation of the histopathological stage of tumours with KP expression has shown that peptide levels decrease with progression of the cancer. High expression has been detected in benign and radial growth phase tumours, with lower expression detected in more advanced clinical stages (Ikeguchi et al., 2003; Sanchez-Carbayo et al., 2003b; Jiang et al., 2005) although, in contrast to Microarray data from metastatic cell lines (Becker et al., 2005), no association with cell cycle stage was identified (Sanchez-Carbayo et al., 2003b).
KP and placentation
Quantitative PCR and Microarray analysis detected expression of KP and KISS1 (GPR54) in human trophoblasts (Janneau et al., 2002; Bilban et al., 2004). Laser capture microdissection specifically detected KP and KISS1 (GPR54) in villous cytotrophoblasts. Transcriptional expression of KP did not change between early and term placentas, but KISS1 (GPR54) expression was higher in early placentas compared to term placentas coinciding with changes from highly invasive cells early in pregnancy to less invasive cells at term (Janneau et al., 2002). A subsequent Microarray analysis confirmed the higher KISS1 (GPR54) expression in first trimester invasive trophoblasts, when compared to non/low-invasive term cells. This study further localized KISS1 (GPR54) expression to villous and extravillous trophoblasts, with KP only expressed in villous trophoblasts, suggesting both autocrine and paracrine actions (Bilban et al., 2004). In parallel to cancer phenotypes, trophoblast migration was inhibited by KP and was also associated with suppression of MMP-2 and MMP-9 activity (Bilban et al., 2004; Qiao et al., 2005).
Two site-enzyme immunoassay detected normal plasma KP-54 concentrations of approximately 1 fmol ml−1 in males and females, respectively. Measurement of KP-54 throughout pregnancy revealed a 1000-fold increase of KP-54 in the first trimester, with up to a 10 000-fold increase by the third trimester. Levels returned to near baseline by day 5 after partum, suggesting the placenta as the source of KP in pregnancy (Horikoshi et al., 2003). In contrast to expression in invasive extravillious trophoblasts, detected at the mRNA level, immunohistochemical staining of KP-54 in human placenta detected KP-54-like immunoreactivity in the transport trophoblast subtype, the syncytiotrophoblasts, which are responsible for secretion of peptides into the maternal bloodstream. Whether the KP/KISS1 (GPR54) have a direct effect on invasive trophoblasts, or an indirect effect via secretory trophoblasts, or act through both mechanisms to regulate placentation remains to be determined.
The role of KP in placentation and the regulatory association with MMPs led to the hypothesis that they may have an additional role in the pathogenesis of pre-eclampsia. Co-analysis of KP and MMP-9 mRNA expression in trophoblasts of women with pre-eclampsia, compared to normal pregnancy, detected significantly higher KP levels in pre-eclampsia, corresponding with significantly lower MMP-9 expression levels (Qiao et al., 2005). In contrast, a second study identified significantly lower KP mRNA levels in pre-eclampsia (Farina et al., 2006). Further studies are required into the association of KP/KISS1 (GPR54) and pre-eclampsia, but these data suggest that KP/KISS1 (GPR54) contribute to the pathogenesis of this disease.
KISS1 (GPR54) – an unexpected molecular switch for puberty
In 2003, three different groups identified KISS1 (GPR54) as an unexpected molecular switch for puberty (de Roux et al., 2003; Funes et al., 2003; Seminara et al., 2003). Genetic linkage analysis on a consanguineous family, with members who had idiopathic hypogonadotrophic hypogonadism (IHH), identified a homozygous leucine148 to serine148 (L148S by convention) mutation in the receptor gene (Seminara et al., 2003) and subsequently the heterozygous mutations R331X (X: undetermined amino acid) and X399R in a separate family with IHH. Subjects with these mutations have low gonadotrophin levels and a complete or partial absence of luteinizing hormone (LH) pulsations and do not undergo puberty, although they do respond to treatment with replacement gonadotropin-releasing hormone (GnRH) (Seminara et al., 2003; Aparicio, 2005). At the same time, a homozygous deletion of 155 nucleotides spanning intron 4 and part of exon 5 and a mis-sense homozygous point mutation L102P was identified in different probands (de Roux et al., 2003). Since the initial mutations were described, additional mis-sense mutations in the KISS1 (GPR54) receptor resulting in IHH have been detected including C223R and R297L (Semple et al., 2005).
The KISS1 (GPR54) knockout mice provided an elegant example of a phenocopy syndrome between humans and mice (MacDonald, 2004). In parallel to human studies, Seminara et al. generated KISS1 (GPR54)−/− mice. Male mice had greatly reduced testes size, hypoplastic Leydig cells, spermatogenic arrest and lacked development of secondary sex glands. Female mice had small vaginal openings, were sterile and the oestrous cycle was absent. Ovary size and uterine horns were greatly reduced and ovaries contained only early follicles, no Graafian follicles or corpora lutea. A third group studying KISS1 (GPR54) and puberty simultaneously developed knockout mice, which exhibited the same phenotype as those used by Seminara et al. (Funes et al., 2003). Hormone profiling of KISS1 (GPR54)−/− mice detected striking similarities to the human syndrome, with low gonadotrophin levels, but retaining the ability to respond to exogenous GnRH. The results in human and mouse led to the hypothesis that KP have an effect on secretion or processing of GnRH.
KP and GnRH release
The identification of KP as a molecular switch for puberty attracted several groups to try to determine the mechanisms for this novel pathway. The first group to begin unravelling the signalling pathway showed that injection of KP-10 and KP-54 directly into the lateral cerebral ventricle of the mouse brain potently stimulated LH and follicle-stimulating hormone (FSH) secretion, an effect that could be blocked by pre-treatment with the GnRH antagonist acyline (Gottsch et al., 2004). This finding has since been confirmed in adult and prepubertal rats (Matsui et al., 2004; Navarro et al., 2004; Thompson et al., 2004; Irwig et al., 2005), sheep (Messager et al., 2005), monkeys (Shahab et al., 2005) and normal human males (Dhillo et al., 2005). In addition to LH/FSH surges induced by KP, sex steroid surges have also been identified in human and rat (Dhillo et al., 2005). Limited evidence suggests direct effects of the KP on the gonadotrophs (Thompson et al., 2004), however it is more likely that the KP stimulate the gonadotrophs via the GnRH neurones.
In contrast, continuous administration of KP-10 to the juvenile male monkey resulted in an initial acute stimulation of LH secretion followed by a selective and KISS1 (GPR54) receptor-specific desensitization (Seminara et al., 2006). The reduction in responsiveness to KP associated with receptor desensitization was accompanied by downregulation of GnRH/LH secretion, a finding that may have clinical implications for the treatment of hypogonadotrophic states by GnRH (Seminara et al., 2006). Receptor desensitization was also observed following chronic infusion of KP-54 in adult male rats, again leading to downregulation of LH secretion and resulting in testicular degeneration, possibly owing to changes in testicular blood flow (Thompson et al., 2006).
KP neurones in the brain
Approximately 75% of GnRH neurones co-express KISS1 (GPR54) (Irwig et al., 2005), a finding that has been confirmed in sheep, where intracerebral injection of KP resulted in direct release of GnRH into the cerebrospinal fluid (Messager et al., 2005). Prominent regions of KP expression in the brain are the arcuate nucleus (Arc), periventricular nucleus (PeN), anteroventral periventricular nucleus (AVPV), with lower levels in the anterodorsal preoptic area, and bed nucleus of the stria terminalis (Navarro et al., 2004; Smith et al., 2005a, 2005b).
Navarro et al. (2004) showed that the total KP and KISS1 (GPR54) mRNA levels in female and male rat hypothalamus are inhibited by oestrogen and testosterone, respectively. Two parallel studies in female and male mouse brain confirmed and extended this finding. Comparison of mRNA expression in male mice, which were intact, castrated or castrated with testosterone replacement, detected differential regulation of KP mRNA expression in different brain regions (Smith et al., 2005b). In the Arc, castration resulted in increased KP mRNA and reversal by oestrogen, dihydrotestosterone (DHT) or testosterone. The majority of KP expressing cells also expressed the oestrogen receptor-α (ERα) or androgen receptor (AR), and in cells expressing either mutated ERα or AR testosterone continued to regulate KP expression. The ERβ was not shown to have any role in the KP cascade. These data suggest that in the Arc, KP neurones are key mediators of the negative feedback loop from testosterone to gonadotrophin secretion (Smith et al., 2005b). In the AVPV and PeN the opposite effect was observed, with a lowering of KP mRNA, which was also rescued by testosterone. In further contrast to the Arc, KP effects in AVPV and PeN are mediated only by ER as oestrogen restores KP expression, while DHT does not. In these regions ERβ may have a role, as testosterone was still able to mediate its effects (Smith et al., 2005b).
In ovary intact, ovariectomized and ovariectomized plus oestrogen-treated female mice, the same pattern as in male mice was observed in the Arc – raised KP in ovariectomized mice that was rescued by oestrogen treatment (Smith et al., 2005a). AVPV and PeN also had reduced KP expression in ovariectomized animals, that could be rescued by oestrogen and which appeared to be regulated by ERα. The AVPV is sexually dimorphic, with significantly more KP neurones in females and a suggested role in the preovulatory surge (Smith et al., 2005a). The functional significance of these differing regulatory pathways of KP in the Arc and AVPV remains to be determined (Dungan et al., 2006). In mice, ovulation is driven by a GnRH/LH surge at proestrous, which is regulated by oestrogen-positive feedback. As GnRH neurons do not express ERα this must occur by oestrogen actions upon ERα-expressing neuronal afferents to GnRH neurons (Wintermantel et al., 2006). These neuronal afferents have been proposed to exist within the AVPV. Levels of KP mRNA peak in the AVPV of adult female rats at proestrous, while they are at their lowest point in the Arc. This expression pattern of KP mRNA at proestrous, combined with the expression of ERα mRNA in most KP neurons, has lead to the suggestion that KP neurons in the AVPV play a role in mediating the effects of oestrogen on the generation of the preovulatory GnRH/LH surge (Smith et al., 2006b). The influence of KP neurons in the AVPV on the regulation of ovulation by oestrogen may explain the sexual differentiation of KiSS-1 gene expression in the AVPV but not the Arc (Clarkson and Herbison, 2006; Kauffman et al., 2007).
During lactation the cyclic pattern of oestrous is inhibited, primarily due to the suppression of pulsatile GnRH/LH surges. KP mRNA was significantly lower in the Arc of lactating compared to non-lactating rats, while levels in the AVPV of both sets of animals were continuously low. However, KISS1 (GPR54) mRNA levels were significantly lower in the AVPV of lactating than non-lactating rats but were unchanged in the Arc (Yamada et al., 2007). Changes in mRNA expression of KP/KISS1 (GPR54) did not alter the LH secretory response to KP-54 at sub-nanomolar levels, although an earlier study did find reduced sensitivity to KP-10 during lactation when administered at nanomolar but not sub-nanomolar levels (Roa et al., 2006). Therefore it is probable that the suckling stimulus-induced inhibition of KP/KISS1 (GPR54) expression in the Arc is involved in the suppression of LH secretion in lactating rats (Yamada et al., 2007).
The signalling pathway from KP administration to GnRH secretion via KISS1 (GPR54) has been shown to involve upregulation of the transcription factor associated with neuronal stimulation, Fos (Matsui et al., 2004; Irwig et al., 2005; Smith et al., 2005a, 2005b).
Direct evidence for KP as a molecular switch for puberty
Evidence of the activation of the GnRH cascade by the KP and feedback on the expression of KP in the forebrain by gonadal steroids strongly indicate a role in puberty. To expand on these findings, the role of KP as gatekeepers of puberty has been directly investigated (Dungan et al., 2006). Upregulation of KP in puberty has been shown in the hypothalamus and AVPV of rats and mice (Navarro et al., 2004; Han et al., 2005) and in the hypothalamus of monkeys (Shahab et al., 2005). Central administration of KP to prepubertal female rats advanced vaginal opening, suggesting KP could directly initiate the onset of puberty. Some discrepancy exists, as KISS1 (GPR54) receptors were not upregulated in males during puberty (Han et al., 2005), although there was a potential increase in efficacy of the KP in these animals at puberty. In several species, reproduction is controlled photoperiodically (Revel et al., 2006). The role of KP in timing of puberty made them likely modulators of the photoperiodic control. In Syrian hamsters, where reproduction is promoted by long days and inhibited by short days, KP were significantly lower in short-day animals, an effect reliant on melatonin signalling, as pineal gland ablation prevented the downregulation. In the Arc of ewe, brain KP mRNA expression was lower during anoestrus, due to a non-steroid-dependent seasonal effect, further suggesting a role for KP in control of seasonal changes in reproductive function (Smith et al., 2007). Although the KP pathway is a prerequisite for puberty, it is unlikely to be the sole gatekeeper, requiring interaction with numerous other factors for puberty to commence (Dungan et al., 2006).
KP and the oestrous cycle
The stimulation of the gonadotrophin axis by the KP suggested possible involvement in the positive feedback loop between oestrogen, GnRH and LH and regulation of the menstrual cycle. Subcutaneous administration of KP-54 induced ovulation in prepubertal female rats, which had been treated with gonadotrophin to induce follicle maturation (Matsui et al., 2004). In extracts of the whole hypothalamus, KP and KISS1 (GPR54) mRNA expression changed as a function of the oestrous cycle with KP expression being at its nadir at proestrus and it is highest at dioestrus (Navarro et al., 2004). However, it was later shown that expression of KP/ KISS1 (GPR54) is at its highest in the AVPV at proestrus, when it is also at its lowest in the Arc (Smith et al., 2005a). Additionally, in the ovary maximum levels of KP were identified at proestrus, with levels remaining low throughout the rest of the cycle with the exception of a transient increase at dioestrus (Castellano et al., 2006a). No changes in KISS1 (GPR54) were detected.
Functionally, injection of KP throughout the oestrous cycle induced LH secretion and maximal responses were achieved at oestrus (Castellano et al., 2006a; Roa et al., 2006). In the seminal work of Kinoshita et al. (2005), immunoneutralization of local metastin action in the preoptic area completely abolished the proestrus LH surge and inhibited oestrus cyclicity. Therefore, increased KP/KISS1 (GPR54) expression is critical for positive feedback in the GnRH cascade and for ovulation. Subsequently, it has been suggested that expression of KP/KISS1 (GPR54) in the AVPV mediates the process of the GnRH surge at proestrus and ovulation, whereas KISS1 (GPR54) neurons in the Arc are likely to play a role in the negative feedback regulation of GnRH/gonadotrophin secretion (Smith et al., 2005a, 2005b). This suggestion is reinforced by the findings of Wintermantel et al. (2006) identifying a population of oestrogen-sensitive neurons in the AVPV communicating directly with GnRH neurons. The observation that KISS1 (GPR54) expression is increased in the AVPV at the time of the GnRH/LH surge, coupled with the abolition of the proestrus LH surge by the immunoneutralization of local metastin action in the preoptic area, leads to the conclusion that KISS1 (GPR54) neurons in the AVPV drive the event of ovulation.
Immunocytochemistry (ICC) data identified KP immunoreactivity in specific ovarian compartments including theca of growing and pre-ovulatory follicles, theca and granulosa-lutein cells of corpus luteum and interstitial gland, the ovarian surface epithelium and the oocyte. Interestingly, the staining pattern also changed along the oestrous cycle, with absence of expression in oestrous to early proestrous granulosa cells, but detection in late proestrous granulosa cells (Castellano et al., 2006a). The MMPs also have differential expression along the oestrous cycle, facilitating follicular breakdown during the periovulatory period. A possible mechanism of the direct action of KP on ovaries is inhibition of MMPs, to prevent unregulated proteolysis of remaining ovarian tissue after follicular breakdown (Castellano et al., 2006a).
Direct effects of KP on the testes
In addition to the indirect effects the KP have on the testes via GnRH expression, direct effects in the testes have been shown (Kotani et al., 2001; Ohtaki et al., 2001; Funes et al., 2003; Thompson et al., 2004). Continuous chronic administration of KP in male rats resulted in decreased testicular weight and degeneration of the seminiferous tubules, leading to the hypothesis that KP may alter testicular blood flow (Thompson et al., 2004).
KP – a role in diabetes, obesity and cardiovascular disease?
In women of reproductive age, polycystic ovarian syndrome (PCOS) is a common syndrome, which is associated with infertility, increased LH levels and increased resistance to insulin. Therefore, Panidis et al. (2006) sought to investigate potential correlation between PCOS and the KP by comparing KP levels of normal weight women with PCOS, obese women with PCOS and obese controls. In this study, normal weight women with PCOS had significantly higher KP-54 levels and were less insulin resistant than obese women with PCOS (Panidis et al., 2006). Plasma KP levels were also negatively correlated with body mass index and indices of insulin resistance.
KP and KISS1 (GPR54) have been detected in pancreas (Kotani et al., 2001; Muir et al., 2001; Ohtaki et al., 2001), which is a key regulator of whole-body homeostasis. In mouse islets of Langerhans both KISS1 (GPR54) and KP were detected in α- and β-cells, but not in the exocrine pancreatic cells (Hauge-Evans et al., 2006). In functional experiments, exposure of human and mouse islets to KP did not affect basal rate of insulin secretion, but caused a stimulation of glucose-induced insulin secretion. KISS1 (GPR54) was also detected in insulin-secreting mouse β-islet cell lines although, in contrast to primary tissue, KP exposure inhibited insulin secretion at basal and stimulated glucose levels (Hauge-Evans et al., 2006) in these cells. As hypogonadism is common in uncontrolled diabetes, a further study sought to elucidate the potential role of KP in this phenomenon. In streptozotocin-induced diabetic rats, where the gonadotrophic axis is attenuated, KP administration evoked LH and testosterone bursts (Castellano et al., 2006b). KiSS-1 gene expression was severely decreased in these rats and could be rescued by infusion of leptin, but not insulin.
Leptin is a satiety factor, which is produced by adipocytes and acts on the forebrain, including in the Arc where expression of KISS1 (GPR54) has been described (Castellano et al., 2005; Smith et al., 2006a). KP reduced the decline in gonadotrophin secretion that is observed in rats treated with leptin antibodies (Castellano et al., 2005). Comparison of castrated wild-type mice to leptin-deficient ob/ob mice identified a significant reduction in KP mRNA in the Arc of ob/ob mice. When treated with leptin KP mRNA levels in ob/ob mice were increased, although not fully restored (Smith et al., 2006a). Additionally, 40% of KP mRNA-expressing cells in the Arc also expressed the leptin receptor mRNA Ob-Rb, suggesting that leptin is a direct regulator of KP neurones.
The endogenous ligand of the growth hormone secretagogue receptor, ghrelin (Kojima et al., 1999), is a regulator of energy homeostasis and reproduction. Ghrelin has been shown to suppress LH secretion in both rats and monkeys. Therefore, to further elucidate effects of ghrelin on LH secretion, KP and ghrelin were co-administered. Although ghrelin did not significantly modify the magnitude of the acute stimulatory effects of KP on LH secretion, it did significantly shorten the duration and net magnitude of the response (Martini et al., 2006). It was therefore speculated that in conditions of hyperghrelinemia, such as observed in low body mass index, the LH releasing ability of the KP may be negatively impacted (Martini et al., 2006).
Diabetes and obesity are associated with cardiovascular disease and, furthermore, tumour metastasis and placentation are processes involving angiogenesis, leading to the hypothesis that the KP may function as novel cardiovascular transmitters. In human, vasculature KISS1 (GPR54) has a restricted localization to smooth muscle of vessels with the same developmental origins, umbilical vein, coronary artery and aorta (Mead et al., 2006). Interestingly, KISS1 (GPR54) and the KP also localized to cells within the atherosclerotic plaque of coronary artery. In isolated rings of coronary artery and umbilical vein KP-10, KP-13 and KP-54 acted as vasoconstrictors with comparable potencies and maximum responses. The local detection and vasoconstrictor action of the KP in human vasculature suggests that they may act as novel paracrine vascular transmitters at the KISS1 (GPR54) receptor. Discrete localization of receptor to vessels prone to atherosclerosis also implicates this receptor system in the pathophysiology of cardiovascular disease (Mead et al., 2006).
In addition to roles in puberty, metastasis and placentation, a role for KP is emerging in whole-body homeostasis, by novel interactions with peptides that have well characterized roles in this process, such as insulin, leptin and ghrelin.
Synthetic agonists
Alanine and D-amino acid replacement scanning of KP-10 revealed that the final five amino acids are essential for agonist activity at KISS1 (GPR54). Synthetic derivatives based on the structure of KP-10, of approximately five amino acids length, showed high affinity and comparable potency to KP-10. Further structure–activity relationship studies on these compounds identified H-Amb-Nal(2)-Glycine-Leucine-Arginine-Tryptophan-NH2 (Amb: 4-(aminomethyl)benzoic acid; Nal(2): 3-(2-naphthyl)alanine) as the most potent KISS1 (GPR54) agonist reported to date. Further modification of synthetic pentapeptide analogues revealed that in a synthetic agonist (H-Amb-Phenylalanine-Glycine-Leucine-Arginine-Tryptophan-NH2) which shares four amino acids with the C terminus of the KP, the phenylalanine-glycine bond is required in the trans-amide conformer for biological activity (Tomita et al., 2007). A pharmacophore model derived from KP-13 structure–activity relationship studies showed that phenylalanine9, arginine12 and phenylalanine13 are the key amino acids for KP function. Using this model, small molecule compounds with agonist activity at KISS1 (GPR54) were discovered from a corporate library; however, they had significantly lower potency and affinity than the native peptide (Orsini et al., 2007). No antagonists at this receptor have currently been described.
Conclusions and future directions
The pairing of the KP with the KISS1 (GPR54) receptor has received growing attention since the description of the receptor as a molecular switch for puberty. Mechanisms underlying the role of KISS1 (GPR54) in puberty are the focus of the majority of emerging reports into this receptor system. A significant body of evidence across several species now suggests that KISS1 (GPR54) activation is a critical point in the commencement of puberty, although further investigation is required to fully characterize the interaction between KP and the GnRH cascade. Caution must be exercised when drawing specific inferences from the animal data summarized in this review on the role of the KP in human reproduction, as differences in the physiological mechanisms regulating reproduction exist between species. Synthetic agonists targeting KISS1 (GPR54) may represent novel therapeutic agents for the treatment of hypogonadotrophic hypogonadism in some affected individuals, although, prior to consideration as a drug target the other significant functions of the KP including their role in placentation, anti-metastatic effect and vasoconstrictor action must be further investigated. The diverse multifunctional nature of the KP is beginning to unravel. The unexpected role of these peptides in puberty has given rise to a number of important questions that remain to be answered to elucidate the extent to which KP regulate the timing of puberty. However, in answering these questions other important areas of emerging pharmacology and physiology of this receptor and peptide should not be overlooked.
Acknowledgments
We thank the British Heart Foundation for their support. Emma J Mead was the recipient of the 2003 AJ Clark studentship of the British Pharmacological Society.
Abbreviations
- Amb
4-(aminomethyl)benzoic acid
- AR
androgen receptor
- Arc
arcuate nucleus
- AVPV
anteroventral periventricular nucleus
- DHT
dihydrotestosterone
- ER
oestrogen receptor
- ERK
extracellular signal-regulated kinase
- FCS
foetal calf serum
- FSH
follicle-stimulating hormone
- GnRH
gonadotropin-releasing hormone
- GPCR
G-protein-coupled receptor
- KISS1R
KISS1 receptor gene
- IHH
idiopathic hypogonadotrophic hypogonadism
- IUPHAR
International Union of Pharmacology
- JNK
c-Jun N-terminal kinase
- KiSS-1
kisspeptin gene
- KP
kisspeptins
- LH
luteinizing hormone
- MAPK
mitogen-activated protein kinase
- MMP
matrix metalloproteinase
- Nal(2)
3-(2-naphthyl)alanine
- NF-κB
nuclear factor-κB
- PCOS
polycystic ovarian syndrome
- PeN
periventricular nucleus
- VEGF
vascular endothelial growth factor
Conflict of interest
The authors state no conflict of interest.
References
- Aparicio S. Kisspeptins and GPR54 – the new biology of the mammalian GnRH axis. Cell Metab. 2005;1:293–296. doi: 10.1016/j.cmet.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Becker JA, Mirjolet JF, Bernard J, Burgeon E, Simons MJ, Vassart G, et al. Activation of GPR54 promotes cell cycle arrest and apoptosis of human tumor cells through a specific transcriptional program not shared by other Gq-coupled receptors. Biochem Biophys Res Commun. 2005;326:677–686. doi: 10.1016/j.bbrc.2004.11.094. [DOI] [PubMed] [Google Scholar]
- Bilban M, Ghaffari-Tabrizi N, Hintermann E, Bauer S, Molzer S, Zoratti C, et al. Kisspeptin-10, a KiSS-1/metastin-derived decapeptide, is a physiological invasion inhibitor of primary human trophoblasts. J Cell Sci. 2004;117:1319–1328. doi: 10.1242/jcs.00971. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Gaytan M, Roa J, Vigo E, Navarro VM, Bellido C, et al. Expression of KiSS-1 in rat ovary: putative local regulator of ovulation. Endocrinology. 2006a;147:4852–4862. doi: 10.1210/en.2006-0117. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Navarro VM, Fernandez-Fernandez R, Nogueiras R, Tovar S, Roa J, et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146:3917–3925. doi: 10.1210/en.2005-0337. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Navarro VM, Fernandez-Fernandez R, Roa J, Vigo E, Pineda R, et al. Expression of hypothalamic KiSS-1 system and rescue of defective gonadotropic responses by kisspeptin in streptozotocin-induced diabetic male rats. Diabetes. 2006b;55:2602–2610. doi: 10.2337/db05-1584. [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements MK, McDonald TP, Wang R, Xie G, O'Dowd BF, George SR, et al. FMRFamide-related neuropeptides are agonists of the orphan G-protein-coupled receptor GPR54. Biochem Biophys Res Commun. 2001;284:1189–1193. doi: 10.1006/bbrc.2001.5098. [DOI] [PubMed] [Google Scholar]
- Davenport AP, Mead EJ.KiSS1 – derived peptide receptor 2005. IUPHAR Receptor database ()
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar DK, Naora H, Kubota H, Maruyama R, Yoshimura H, Tonomoto Y, et al. Downregulation of KiSS-1 expression is responsible for tumor invasion and worse prognosis in gastric carcinoma. Int J Cancer. 2004;111:868–872. doi: 10.1002/ijc.20357. [DOI] [PubMed] [Google Scholar]
- Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, et al. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90:6609–6615. doi: 10.1210/jc.2005-1468. [DOI] [PubMed] [Google Scholar]
- Dungan HM, Clifton DK, Steiner RA. Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology. 2006;147:1154–1158. doi: 10.1210/en.2005-1282. [DOI] [PubMed] [Google Scholar]
- Farina A, Sekizawa A, Purwosunu Y, Rizzo N, Banzola I, Concu M, et al. Quantitative distribution of a panel of circulating mRNA in preeclampsia versus controls. Prenat Diagn. 2006;26:1115–1120. doi: 10.1002/pd.1562. [DOI] [PubMed] [Google Scholar]
- Foord SM, Bonner TI, Neubig RR, Rosser EM, Pin JP, Davenport AP, et al. International Union of Pharmacology. XLVI. G protein-coupled receptor list. Pharmacol Rev. 2005;57:279–288. doi: 10.1124/pr.57.2.5. [DOI] [PubMed] [Google Scholar]
- Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, et al. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–1363. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- Goldberg SF, Miele ME, Hatta N, Takata M, Paquette-Straub C, Freedman LP, et al. Melanoma metastasis suppression by chromosome 6: evidence for a pathway regulated by CRSP3 and TXNIP. Cancer Res. 2003;63:432–440. [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms JF, Welch DR, Miele ME. KISS1 metastasis suppression and emergent pathways. Clin Exp Metastasis. 2003;20:11–18. doi: 10.1023/a:1022530100931. [DOI] [PubMed] [Google Scholar]
- Hauge-Evans AC, Richardson CC, Milne HM, Christie MR, Persaud SJ, Jones PM. A role for kisspeptin in islet function. Diabetologia. 2006;49:2131–2135. doi: 10.1007/s00125-006-0343-z. [DOI] [PubMed] [Google Scholar]
- Hesling C, D'Incan M, Mansard S, Franck F, Corbin-Duval A, Chevenet C, et al. In vivo and in situ modulation of the expression of genes involved in metastasis and angiogenesis in a patient treated with topical imiquimod for melanoma skin metastases. Br J Dermatol. 2004;150:761–767. doi: 10.1111/j.0007-0963.2004.05898.x. [DOI] [PubMed] [Google Scholar]
- Hori A, Honda S, Asada M, Ohtaki T, Oda K, Watanabe T, et al. Metastin suppresses the motility and growth of CHO cells transfected with its receptor. Biochem Biophys Res Commun. 2001;286:958–963. doi: 10.1006/bbrc.2001.5470. [DOI] [PubMed] [Google Scholar]
- Horikoshi Y, Matsumoto H, Takatsu Y, Ohtaki T, Kitada C, Usuki S, et al. Dramatic elevation of plasma metastin concentrations in human pregnancy: metastin as a novel placenta-derived hormone in humans. J Clin Endocrinol Metab. 2003;88:914–919. doi: 10.1210/jc.2002-021235. [DOI] [PubMed] [Google Scholar]
- Ikeguchi M, Hirooka Y, Kaibara N. Quantitative reverse transcriptase polymerase chain reaction analysis for KiSS-1 and orphan G-protein-coupled receptor (hOT7T175) gene expression in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2003;129:531–535. doi: 10.1007/s00432-003-0469-z. [DOI] [PubMed] [Google Scholar]
- Ikeguchi M, Yamaguchi K, Kaibara N. Clinical significance of the loss of KiSS-1 and orphan G-protein-coupled receptor (hOT7T175) gene expression in esophageal squamous cell carcinoma. Clin Cancer Res. 2004;10:1379–1383. doi: 10.1158/1078-0432.ccr-1519-02. [DOI] [PubMed] [Google Scholar]
- International Human Genome Sequencing Consortium Finishing the euchromatic sequence of the human genome. Nature. 2004;431:931–945. doi: 10.1038/nature03001. [DOI] [PubMed] [Google Scholar]
- Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, et al. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2005;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- Janneau JL, Maldonado-Estrada J, Tachdjian G, Miran I, Motte N, Saulnier P, et al. Transcriptional expression of genes involved in cell invasion and migration by normal and tumoral trophoblast cells. J Clin Endocrinol Metab. 2002;87:5336–5339. doi: 10.1210/jc.2002-021093. [DOI] [PubMed] [Google Scholar]
- Jiang T, Zhang SL, Lin B, Meng LR, Gao H. Expression and clinical significance of KISS-1 and GPR54 mRNA in endometrial carcinoma. Zhonghua Zhong Liu Za Zhi. 2005;27:229–231. [PubMed] [Google Scholar]
- Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, et al. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;184:1774–1783. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, et al. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146:4431–4436. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- Lee DK, Nguyen T, O'Neill GP, Cheng R, Liu Y, Howard AD, et al. Discovery of a receptor related to the galanin receptors. FEBS Lett. 1999;446:103–107. doi: 10.1016/s0014-5793(99)00009-5. [DOI] [PubMed] [Google Scholar]
- Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, et al. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88:1731–1737. doi: 10.1093/jnci/88.23.1731. [DOI] [PubMed] [Google Scholar]
- Lee JH, Welch DR. Suppression of metastasis in human breast carcinoma MDA-MB-435 cells after transfection with the metastasis suppressor gene, KiSS-1. Cancer Res. 1997;57:2384–2387. [PubMed] [Google Scholar]
- MacDonald MLE. Mutations in a G protein-coupled receptor cause hypogonadotrophic hypogonadism in humans and mice. Clin Genet. 2004;65:177–182. [Google Scholar]
- Maguire JJ, Davenport AP. Regulation of vascular reactivity by established and emerging GPCRs. Trends Pharmacol Sci. 2005;26:448–454. doi: 10.1016/j.tips.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Martini AC, Fernandez-Fernandez R, Tovar S, Navarro VM, Vigo E, Vazquez MJ, et al. Comparative analysis of the effects of ghrelin and unacylated ghrelin on luteinizing hormone secretion in male rats. Endocrinology. 2006;147:2374–2382. doi: 10.1210/en.2005-1422. [DOI] [PubMed] [Google Scholar]
- Masui T, Doi R, Mori T, Toyoda E, Koizumi M, Kami K, et al. Metastin and its variant forms suppress migration of pancreatic cancer cells. Biochem Biophys Res Commun. 2004;315:85–92. doi: 10.1016/j.bbrc.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun. 2004;320:383–388. doi: 10.1016/j.bbrc.2004.05.185. [DOI] [PubMed] [Google Scholar]
- Mead EJ, Maguire JJ, Kuc RE, Davenport AP. Kisspeptins are novel potent vasoconstrictors in humans, with a discrete localization of their receptor GPR54, to atherosclerosis prone vessels. Endocrinology. 2006;148:140–147. doi: 10.1210/en.2006-0818. [DOI] [PubMed] [Google Scholar]
- Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miele ME, Robertson G, Lee JH, Coleman A, McGary CT, Fisher PB, et al. Metastasis suppressed, but tumorigenicity and local invasiveness unaffected, in the human melanoma cell line MelJuSo after introduction of human chromosomes 1 or 6. Mol Carcinog. 1996;15:284–299. doi: 10.1002/(SICI)1098-2744(199604)15:4<284::AID-MC6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Mitchell DC, Abdelrahim M, Weng J, Stafford LJ, Safe S, Bar-Eli M, et al. Regulation of KiSS-1 metastasis suppressor gene expression in breast cancer cells by direct interaction of transcription factors activator protein-2alpha and specificity protein-1. J Biol Chem. 2006;281:51–58. doi: 10.1074/jbc.M506245200. [DOI] [PubMed] [Google Scholar]
- Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276:28969–28975. doi: 10.1074/jbc.M102743200. [DOI] [PubMed] [Google Scholar]
- Mullins LJ, Mullins JJ. Large transgenes reveal their secrets. Focus on ‘differential expression of the closely linked KISS1, REN, and FLJ10761 genes in transgenic mice'. Physiol Genomics. 2004;17:1–3. doi: 10.1152/physiolgenomics.00019.2004. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, et al. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- Orsini MJ, Klein MA, Beavers MP, Connolly PJ, Middleton SA, Mayo KH. Metastin (KiSS-1) mimetics identified from peptide structure-activity relationship-derived pharmacophores and directed small molecule database screening. J Med Chem. 2007;50:462–471. doi: 10.1021/jm0609824. [DOI] [PubMed] [Google Scholar]
- Panidis D, Rousso D, Koliakos G, Kourtis A, Katsikis I, Farmakiotis D, et al. Plasma metastin levels are negatively correlated with insulin resistance and free androgens in women with polycystic ovary syndrome. Fertil Steril. 2006;85:1778–1783. doi: 10.1016/j.fertnstert.2005.11.044. [DOI] [PubMed] [Google Scholar]
- Qiao C, Wang CH, Shang T, Lin QD. Clinical significance of KiSS-1 and matrix metalloproteinase-9 expression in trophoblasts of women with preeclampsia and their relation to perinatal outcome of neonates. Zhonghua Fu Chan Ke Za Zhi. 2005;40:585–590. [PubMed] [Google Scholar]
- Revel FG, Saboureau M, Masson-Pevet M, Pevet P, Simonneaux V, Horvath TL, et al. Kiss-1 is a likely candidate for the photoperiodic control of reproduction. Chronobiol Int. 2006;23:277–287. doi: 10.1080/07420520500521939. [DOI] [PubMed] [Google Scholar]
- Ringel MD, Hardy E, Bernet VJ, Burch HB, Schuppert F, Burman KD, et al. Metastin receptor is overexpressed in papillary thyroid cancer and activates MAP kinase in thyroid cancer cells. J Clin Endocrinol Metab. 2002;87:2399. doi: 10.1210/jcem.87.5.8626. [DOI] [PubMed] [Google Scholar]
- Roa J, Vigo E, Castellano JM, Navarro VM, Fernandez-Fernandez R, Casanueva FF, et al. Hypothalamic expression of KiSS-1 system and gonadotropin-releasing effects of kisspeptin in different reproductive states of the female Rat. Endocrinology. 2006;147:2864–2878. doi: 10.1210/en.2005-1463. [DOI] [PubMed] [Google Scholar]
- Sanchez-Carbayo M, Belbin TJ, Scotlandi K, Prystowsky M, Baldini N, Childs G, et al. Expression profiling of osteosarcoma cells transfected with MDR1 and NEO genes: regulation of cell adhesion, apoptosis, and tumor suppression-related genes. Lab Invest. 2003a;83:507–517. doi: 10.1097/01.lab.0000064702.63200.94. [DOI] [PubMed] [Google Scholar]
- Sanchez-Carbayo M, Capodieci P, Cordon-Cardo C. Tumor suppressor role of KiSS-1 in bladder cancer: loss of KiSS-1 expression is associated with bladder cancer progression and clinical outcome. Am J Pathol. 2003b;162:609–617. doi: 10.1016/S0002-9440(10)63854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminara SB, Dipietro MJ, Ramaswamy S, Crowley WF, Jr, Plant TM. Continuous human metastin 45–54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male Rhesus monkey (Macaca mulatta): a finding with therapeutic implications. Endocrinology. 2006;147:2122–2126. doi: 10.1210/en.2005-1550. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Semple RK, Achermann JC, Ellery J, Farooqi IS, Karet FE, Stanhope RG, et al. Two novel missense mutations in G protein-coupled receptor 54 in a patient with hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2005;90:1849–1855. doi: 10.1210/jc.2004-1418. [DOI] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaki F, Takata M, Hatta N, Takehara K. Loss of expression of the metastasis suppressor gene KiSS1 during melanoma progression and its association with LOH of chromosome 6q16.3–q23. Cancer Res. 2001;61:7422–7425. [PubMed] [Google Scholar]
- Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006a;18:298–303. doi: 10.1111/j.1365-2826.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- Smith JT, Clay CM, Caraty A, Clarke IJ. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology. 2007;148:1150–1157. doi: 10.1210/en.2006-1435. [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005a;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, et al. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005b;146:2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006b;26:6687–6694. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takino T, Koshikawa N, Miyamori H, Tanaka M, Sasaki T, Okada Y, et al. Cleavage of metastasis suppressor gene product KiSS-1 protein/metastin by matrix metalloproteinases. Oncogene. 2003;22:4617–4626. doi: 10.1038/sj.onc.1206542. [DOI] [PubMed] [Google Scholar]
- Thompson EL, Murphy KG, Patterson M, Bewick GA, Stamp GW, Curtis AE, et al. Chronic subcutaneous administration of kisspeptin-54 causes testicular degeneration in adult male rats. Am J Physiol Endocrinol Metab. 2006;291:E1074–E1082. doi: 10.1152/ajpendo.00040.2006. [DOI] [PubMed] [Google Scholar]
- Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, et al. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2004;16:850–858. doi: 10.1111/j.1365-2826.2004.01240.x. [DOI] [PubMed] [Google Scholar]
- Tomita K, Narumi T, Niida A, Oishi S, Ohno H, Fujii N. Fmoc-based solid-phase synthesis of GPR54-agonistic pentapeptide derivatives containing alkene- and fluoroalkene-dipeptide isosteres. Biopolymers. 2007;14:7595–7603. doi: 10.1002/bip.20676. [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone H, Todman MG, et al. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise A, Gearing K, Rees S. Target validation of G-protein coupled receptors. Drug Discov Today. 2002;7:235–246. doi: 10.1016/s1359-6446(01)02131-6. [DOI] [PubMed] [Google Scholar]
- Wisotzkey JD, Neff M, Pandelidis S. Differential expression of the KISS-1 metastasis suppressor gene in colon cancer. Am J Pathol. 1997;151:ST17. [Google Scholar]
- Yamada S, Uenoyama Y, Kinoshita M, Iwata K, Takase K, Matsui H, et al. Inhibition of metastin (Kisspeptin-54)-GPR 54 signaling in the arcuate nucleus-median eminence region during lactation in rats. Endocrinology. 2007;148:2226–2232. doi: 10.1210/en.2006-1529. [DOI] [PubMed] [Google Scholar]
- Yan C, Wang H, Boyd DD. KiSS-1 represses 92-kDa type IV collagenase expression by down-regulating NF-kappa B binding to the promoter as a consequence of Ikappa Balpha-induced block of p65/p50 nuclear translocation. J Biol Chem. 2001;276:1164–1172. doi: 10.1074/jbc.M008681200. [DOI] [PubMed] [Google Scholar]