Abstract
Background and purpose:
Chronic morphine administration produces tolerance in vivo and attenuation of μ opioid receptor (MOR)-mediated G-protein activation measured in vitro, but the relationship between these adaptations is not clear. The present study examined MOR-mediated G-protein activation in the CNS of mice with different levels of morphine tolerance.
Experimental approach:
Mice were implanted with morphine pellets, with or without supplemental morphine injections, to induce differing levels of tolerance as determined by a range of MOR-mediated behaviours. MOR function was measured using agonist-stimulated [35S]guanylyl-5′-O-(γ-thio)-triphosphate ([35S]GTPγS) and receptor binding throughout the CNS.
Key results:
Morphine pellet implantation produced 6-12-fold tolerance in antinociceptive assays, hypothermia and Straub tail, as measured by the ratio of morphine ED50 values between morphine-treated and control groups. Pellet implantation plus supplemental injections produced 25-50-fold tolerance in these tests. In morphine pellet-implanted mice, MOR-stimulated [35S]GTPγS binding was significantly reduced only in the nucleus tractus solitarius (NTS) and spinal cord dorsal horn in tissue sections from morphine pellet-implanted mice. In contrast, MOR-stimulated [35S]GTPγS binding was significantly decreased in most regions examined in morphine pellet+morphine injected mice, including nucleus accumbens, caudate-putamen, periaqueductal gray, parabrachial nucleus, NTS and spinal cord.
Conclusions and implications:
Tolerance and the regional pattern of apparent MOR desensitization were influenced positively by the level of morphine exposure. These results indicate that desensitization of MOR-mediated G-protein activity is more regionally widespread upon induction of high levels of tolerance, suggesting that this response contributes more to high than low levels of tolerance to CNS-mediated effects of morphine.
Keywords: brainstem, spinal cord, opiate, [35S]GTPγS autoradiography, desensitization, downregulation
Introduction
Morphine and other opioids are among the most effective analgesic drugs. However, tolerance develops with chronic use, necessitating escalation of drug doses to achieve equivalent effects (Way et al., 1969; Smith et al., 2003). Acute and chronic effects of most clinically relevant opioids are mediated by the μ opioid receptor (MOR), as confirmed in null mice lacking MOR (Matthes et al., 1996; Tian et al., 1997; Loh et al., 1998). MOR inhibit neurotransmission via pertussis toxin-sensitive G-proteins Gαi/o resulting in inhibition of adenylyl cyclase activity and calcium channels and activation of potassium channels and several intracellular kinases (Childers, 1991; Law et al., 2000). The distribution of MOR in regions including the limbic system, striatum, thalamus, hypothalamus, brainstem and spinal cord is consistent with their in vivo effects on pain, motivation and homeostasis.
Tolerance to opioid-mediated effects has been proposed to result from downregulation of MOR and/or loss of MOR-mediated G-protein activity or effector responses. Studies in cell culture models have demonstrated both of these MOR adaptations after prolonged agonist exposure (Puttfarcken and Cox, 1989; Breivogel et al., 1997). However, studies in the central nervous system (CNS) have shown that MOR levels are generally unchanged or increased in the CNS of chronic morphine-treated animals (Sim-Selley et al., 2000; Stafford et al., 2001; Patel et al., 2002), whereas treatment with highly potent and efficacious compounds such as etorphine produces MOR downregulation (Tao et al., 1987; Stafford et al., 2001; Patel et al., 2002). In contrast, chronic morphine-induced attenuation of MOR-mediated effector responses has been reported, including decreased modulation of adenylyl cyclase (Noble and Cox, 1996; Deng et al., 2001) and potassium channels (Christie et al., 1987). These alterations exhibit region-specific patterns, suggesting that a single mechanism cannot explain tolerance to all morphine-mediated effects. Moreover, differences in chronic administration paradigms and in vitro assay procedures might further influence the results of these studies. The paradigm used to administer chronic morphine can produce different levels of antinociceptive tolerance (Smith et al., 2003), but few studies have provided a comprehensive evaluation of tolerance to various MOR-mediated behaviours and the relationship to changes in MOR function in specific CNS regions.
The effect of chronically administered opioids on MOR-mediated G-protein activation has been directly evaluated by measuring low KmGTPase activity or agonist-stimulated [35S]GTPγS binding in rat CNS. These studies revealed region-specific decreases in MOR-mediated activity, indicative of receptor-G-protein uncoupling or ‘desensitization' (Tao et al., 1993; Sim et al., 1996; Sim-Selley et al., 2000; Maher et al., 2001; Kruzich et al., 2003). In general, greater apparent MOR desensitization has been observed in caudal CNS regions, such as spinal cord, periaqueductal grey (PAG), pontine and medullary nuclei than in forebrain regions, although the physiological basis for this region-dependent MOR adaptation is unclear. One factor that might determine the regional distribution of apparent MOR desensitization is the level of opioid tolerance achieved, but this hypothesis has not been tested directly. In the present study, agonist-stimulated [35S]GTPγS binding and receptor binding were used to examine the effect of different levels of morphine tolerance on MOR-mediated G-protein activation and receptor levels in brain sections or membrane homogenates prepared from treated mice. Behavioural evaluation of several morphine-mediated effects (antinociception, Straub tail, hypothermia) was conducted to provide a comprehensive evaluation of tolerance in multiple neural systems.
Materials and methods
Materials
Male Swiss Webster mice (25–30 g) were obtained from Harlan Laboratories (Indianapolis, IN, USA). All animal procedures were approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University and comply with the European Communities Council Directive of 24 November 1986 (86/609/EEC) to minimize pain and discomfort.
Chronic morphine administration
Mice were treated as previously described (Smith et al., 2003) to produce different levels of antinociceptive tolerance. Mice were anaesthetized with diethyl ether, and a placebo pellet or 75 mg morphine pellet was surgically inserted into a subcutaneous space at the base of the neck. The animals recovered in their home cages where they remained throughout the experiment. Four treatment paradigms were used to produce different levels of tolerance: placebo pellet (control), morphine pellet, placebo pellet plus supplemental saline injections or morphine pellet plus supplemental morphine injections. Supplemental morphine injections were administered as follows: the first injection of 40 mg kg−1 subcutaneously (s.c.) was given 12 h after morphine-pellet implantation. At 24 and 36 h, the mice were injected with an 80 mg kg−1 dose of morphine s.c. At 48 and 60 h the mice were injected with 160 mg kg−1 morphine s.c. Separate groups of mice from the three treatment groups were used to assess [D-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin (DAMGO)-stimulated [35S]GTPγS binding in sections and membranes 72 h after pellet implantation. Because no differences in morphine potency were found between placebo pellet- and placebo pellet+saline-injected groups, placebo-pelleted mice were used as a control in all the in vitro studies. In each case, a cohort group of mice was used to assess in vivo tolerance in the tail withdrawal assay. Unless otherwise indicated, the number of mice for all in vivo measures was 30 each per group: placebo pellet, morphine pellet, placebo pellet+saline injection and morphine pellet+morphine injection. This number included six mice per group for each of four morphine challenge doses and saline vehicle.
Tail withdrawal test
The warm water tail withdrawal apparatus used to assess antinociception was maintained at 56±0.1°C. Before injecting the mice, the base-line (control) latency was determined. Only mice with a control reaction time from 2 to 4 s were used. The average baseline tail withdrawal latency for these experiments was 3.1 s. The test latency after drug treatment was assessed at the appropriate time, and a 10 s maximum cutoff time was imposed to prevent tissue damage. Antinociception was quantified as the percentage of maximum possible effect (%MPE) which was calculated as: %MPE=[(test−control)(10−control)−1] × 100. Percent MPE was calculated for each mouse using six mice per dose. Morphine dose–response curves were generated for calculation of ED50 (half-maximal effective dose) values and 95% confidence limits (CL). These values were calculated using least squares linear regression analysis followed by calculation of 95% CL by Bliss (1967). Tests for parallelism were conducted before calculation of potency ratio values and 95% CL by the method of Colquhoun (1971). A potency ratio value greater than one, with the lower 95% CL greater than one, was considered a significant difference in potency.
Hot-plate test
The hot-plate test was performed as described by O'Callaghan and Holtzman (1975). The mice were first placed on a Syscom Model 35D hot-plate set at 56°C to obtain baseline response latencies before drug administration. The mice were observed for licking either their fore- or hindlimb in response to the heat. The latencies ranged between 5 and 6 s. The mice were tested again at the appropriate time after administration of morphine s.c. A 30 s cutoff was employed in order to prevent tissue damage. Antinociception was quantified according to the method of Harris and Pierson (1964) as the percentage of maximum possible effect (%MPE) which was calculated as: %MPE=[(test latency−control latency)(30–control latency)−1] × 100. Percent MPE was calculated for each mouse using six mice per dose.
Hypothermia measurements
Hypothermia was measured through use of a rectal temperature probe. Baseline temperatures were recorded before test drug administration, measuring approximately 37°C for each mouse. The body temperature was recorded again after administration of morphine s.c., ranging in temperatures from 30 to 36°C. These temperatures were used to calculate the change in body temperature, Tb=test temperature−baseline temperature, as a measure of hypothermia.
Measurement of Straub tail
The mice were observed for the development of morphine-induced Straub tail. The Straub tail reaction was graded using a modified numerical scoring system of Kameyama et al. (1978) as follows: 0=0°, 25%=1–30°, 50%=31–45°, 75%=46–60°, 100%=61–90°. The angle was measured above the horizontal plane of the table. The units were expressed as % Straub tail values.
Agonist-stimulated [35S]GTPγS autoradiography
Animals were decapitated at 72 h and brains were removed and frozen in isopentane at −35°C. Coronal sections (20 μm) were cut on a cryostat maintained at −20°C and collected on gelatin-coated slides. Sections were collected at six levels to include (1) caudate-putamen/nucleus accumbens, (2) thalamus/amygdala, (3) PAG, (4) parabrachial nucleus, (5) nucleus tractus solitarius (NTS), (6) cervical spinal cord. Slides were desiccated overnight at 4°C, and then stored at −80°C until assay. Agonist-stimulated [35S]GTPγS autoradiography was conducted as described previously (Sim et al., 1995; Sim-Selley et al., 2000). Briefly, slides were rinsed in 50 mM Tris-HCl, 3 mM MgCl2, 0.2 mM EGTA (ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′,-tetraacetic acid), 100 mM NaCl, pH 7.4 (assay buffer) at 25°C for 10 min, then incubated for 15 min in TME buffer containing 2 mM GDP and adenosine deaminase (9.5 mU ml−1) at 25°C, to inactivate endogenous adenosine and thereby reduce basal [35S]GTPγS binding. Slides were then incubated in TME buffer with GDP, adenosine deaminase, 0.04 nM [35S]GTPγS and 10 μM DAMGO at 25°C for 2 h. This is a maximally effective concentration of DAMGO for stimulation of [35S]GTPγS binding and is blocked by naloxone (Sim et al., 1995). Following incubation, slides were rinsed twice for 2 min each in Tris buffer (50 mM Tris-HCl, pH 7.4) and once in deionized H2O on ice. Slides were dried overnight and exposed to film for 48 h in the presence of 14C microscales.
Films were digitized with a Sony XC-77 video camera and analysed using the NIH IMAGE program for Macintosh computers. Analysis was conducted by a person unaware of the treatment group, using the atlas of Paxinos and Watson (1986) as a guide, and regions were sampled as follows. Caudate-putamen was selected by outlining the region, using the corpus callosum, lateral septum and anterior commissure as landmarks. Nucleus accumbens was sampled immediately ventral to the caudate-putamen and included both the shell and core subdivisions. The cingulate cortex was sampled at the same level and included all laminae. Thalamus, hypothalamus and amygdala were analysed at the level depicted in Figure 2. The medial thalamus was selected and included midline, intralaminar and medial nuclei. The dorsal medial, ventral medial, arcuate and lateral subdivisions of the hypothalamus were included in the analysis of this region. The cortical, medial, basal and lateral subnuclei of the amygdala were also sampled at this level. Analysis of the PAG included both dorsal and lateral subdivisions and excluded the region ventral to the aqueduct corresponding to the dorsal raphe nucleus. Analysis of the parabrachial nucleus was restricted to the lateral portion and NTS sampling was restricted to the caudal extent that corresponded primarily to the commissural NTS. Spinal cord was collected at the cervical level and only the superficial laminae of the dorsal horn were analysed. Resulting values were expressed as nCi [35S]/g tissue wet weight, and were corrected for [35S] from standards based on incorporation of [35S] into sections of frozen brain paste (Sim et al., 1996).
Data analysis for [35S]GTPγS autoradiography
Net agonist-stimulated [35S]GTPγS binding was calculated by subtracting basal binding (obtained in the absence of agonist) from agonist-stimulated binding. Data are reported as mean±standard error (s.e.) of triplicate sections of brains from 10 mice per treatment group. Statistical comparison between control and morphine-treated mice was performed by analysis of variance (ANOVA) followed by post hoc analysis using Dunnett's or Newman–Keuls multiple comparison tests, where P<0.05 was considered statistically significant.
Agonist-stimulated [35S]GTPγS membrane binding
Mice from the three treatment groups were decapitated and brain and spinal cord were removed. Midbrain-dorsal pons, including PAG, locus coeruleus, interpeduncular nuclei, dorsal and median raphe nuclei and parabrachial nuclei, was dissected from the brains. Tissue was stored at −80°C until assay. On the day of assay, brain regions were thawed and membranes prepared as follows. The tissue was homogenized in approximately 10 volumes of 50 mM Tris-HCl, 3 mM MgCl2, 1 mM EGTA, pH 7.4 (membrane buffer) using a Polytron homogenizer. The homogenate was centrifuged at 48 000 g at 4°C for 10 min, resuspended in 30 ml membrane buffer, centrifuged again at 48 000 g at 4°C for 10 min and finally resuspended in assay buffer. Membrane protein levels were determined by the method of Bradford (1976) using bovine serum albumin as the standard. Agonist-stimulated [35S]GTPγS binding was assayed as described previously (Sim et al., 1995), with slight modification. Membranes were preincubated in 10 mU ml−1 adenosine deaminase at 30°C for 10 min before assay. For assay, membranes (5–8 μg protein) were incubated for 120 min at 30°C in assay buffer containing 0.1 nM [35S]GTPγS, 30 μM GDP with and without varying concentrations of DAMGO. Basal [35S]GTPγS binding was assessed in the presence of GDP and absence of drug. Nonspecific [35S]GTPγS binding was measured in the presence of 20 μM unlabeled GTPγS. The incubation was terminated by rapid filtration under vacuum through Whatman GF/B glass fibre filters, followed by three washes with 3 ml ice-cold 50 mM Tris-HCl, pH 7.2. Bound radioactivity was determined by liquid scintillation spectrophotometry at 95% efficiency for 35S after extraction of the filters in 4 ml Budget Solve scintillation fluid.
[3H]Naloxone saturation binding
Tissue was thawed and membranes were prepared and resuspended in assay buffer as described above. Membranes (40–60 μg) were incubated for 2 h at 30°C with varying concentrations of naloxone (0.3–8 nM) in the presence of 0.5 nM unlabeled naltrindole and 2 nM unlabeled norbinaltorphimine to block the δ opioid receptor (DOR) and κ opioid receptor (KOR), respectively. Nonspecific binding was determined with 10 μM naltrexone. Bound radioactivity was determined by liquid scintillation spectrophotometry at 45% efficiency for 3 h after 1 h shaking of filters in 4 ml of Econosafe scintillation fluid.
Data analysis for membrane binding assays
Where not addressed under previous methods, data are reported as mean±s.e. of at least three separate experiments performed in triplicate. All binding data are reported as specific binding. Net-stimulated [35S]GTPγS binding is defined as agonist-stimulated binding minus basal binding. Percent control binding is defined as [net stimulated [35S]GTPγS binding/maximal net-stimulated [35S]GTPγS binding in control (placebo-P) mice × 100%]. Emax and EC50 or Bmax and KD values were calculated by nonlinear regression analysis of concentration–effect or saturation binding curves, respectively, using JMP software (SAS Institute, Cary, NC, USA). Statistical significance of the data was determined by ANOVA followed by post hoc analysis with Dunnett's multiple comparison test, where P<0.05 was considered statistically significant.
Reagents
[35S]GTPγS (1250 Ci/mmol), [3H]naloxone (67 Ci/mmol) and Kodak X-O-mat film were purchased from PerkinElmer Life Sciences (Boston, MA, USA). Placebo and morphine (75 mg) pellets and DAMGO were provided by the Drug Supply Program of the National Institute on Drug Abuse. Morphine sulphate was obtained from Mallinckrodt (St Louis, MO, USA). Adenosine deaminase, GDP and GTPγS were purchased from Sigma Chemical Co. (St Louis, MO, USA). Tris (hydroxymethyl-aminomethane) and Bio-Rad protein dye were purchased from Bio-Rad (Hercules, CA, USA). Econosafe scintillation fluid was purchased from Research Products International (Mt Prospect, IL, USA). All other chemicals were obtained from Sigma Chemical Co. or Fisher Scientific (Pittsburgh, PA, USA).
Results
Morphine tolerance
Mice were implanted with a 75 mg morphine pellet or placebo pellet, with or without supplemental injections of morphine or saline, as described in Materials and methods. These two morphine administration paradigms have previously been shown to produce approximately a sixfold difference in the development of antinociceptive tolerance to morphine over the same time period (Smith et al., 2003). Mice were observed daily throughout the 72 h treatment period and no overt signs of toxicity or withdrawal were noted. Straub tail and increased locomotor activity were observed within 3 h after implantation of morphine pellets; however, these signs diminished from 24 to 72 h. Administration of 80 and 160 mg kg−1 morphine at 24 and 48 h after pellet implantation resulted in transient increases in Straub tail and locomotor activity, which diminished within 1–2 h post injection. Body weights post-treatment for morphine-pelleted mice were 23.7±0.4 g, and 23.0±0.3 g with supplemental injections, versus 27.4±0.4 g for placebo-pelleted mice at 72 h.
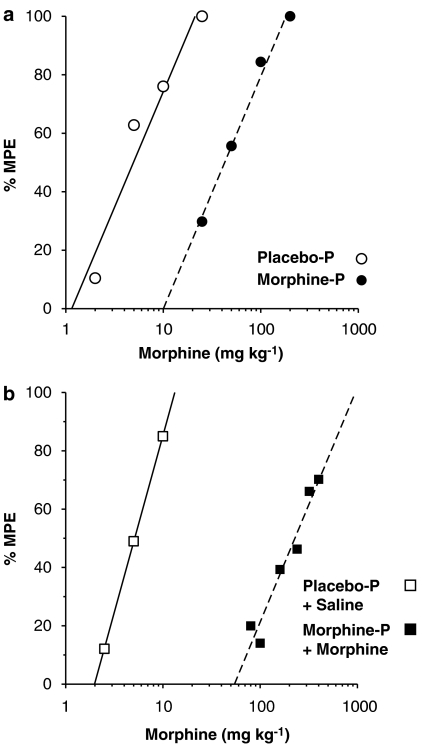
At 72 h after implantation of placebo or morphine pellets, acute morphine antinociception was determined using the 56°C warm water tail withdrawal test. The cohort of mice used for the autoradiographic studies revealed significant levels of tolerance. Morphine was 7.8-fold less potent, with CL of 5.3- to 11.2-fold, in the mice that received a morphine pellet compared to placebo pellet-implanted mice (Figure 1a). The morphine ED50 value (with 95% CL) was 6.7 (5.0–8.9) mg kg−1 in placebo-pelleted mice and was increased to 53.7 (43.1–66.9) mg kg−1 in morphine-pelleted mice. In contrast, morphine was 45.8-fold (31.3- to 66.4-fold) less potent in the mice that received the morphine pellet plus supplemental morphine injections (morphine pellet+morphine) (Figure 1b) than in mice receiving placebo pellet+saline injections. The morphine ED50 value was 5.0 (4.0–6.1) mg kg−1 in placebo pellet+saline-injected mice and was increased to 220.1 (174.2–278.1) mg kg−1 in morphine pellet+morphine-injected mice. The cohort of mice used for membrane studies showed similar results (data not shown), with morphine pellet-implanted mice exhibiting a ∼9-fold rightward shift in the morphine dose–response curve and the morphine pellet+morphine-treated mice exhibiting a ∼50-fold rightward shift (P<0.05 different from placebo pellet+saline). Thus, the morphine pellet+morphine injection paradigm produced greater tolerance development than did the morphine pellet treatment alone, but the levels of tolerance achieved in each group did not significantly differ between cohorts of mice.
Figure 1.
Tolerance to morphine-mediated antinociception. Mice were surgically implanted with placebo pellet (placebo-P) or 75 mg morphine pellet (morphine-P) without (a) or with (b) supplemental saline (placebo-P+saline) or morphine injections (morphine-P+morphine). Seventy-two hours later, morphine was injected s.c. and tail-withdrawal latencies were obtained 30 min after injection, using the warm water (56°C) tail withdrawal procedure. Each curve represents six mice per dose per group.
Morphine tolerance was further assessed in dependent measures with more prominent supraspinal components than the tail withdrawal response, which is a spinal reflex, using additional cohorts of mice. For these experiments, the hot-plate test for antinociception, rectal temperature to measure hypothermia and Straub tail induction were examined. Results were similar to those obtained with tail withdrawal. Morphine tolerance in morphine pellet-treated mice ranged from approximately 6- to 12-fold, with the least tolerance apparent in Straub tail and the greatest in hypothermia, although there were no significant differences in potency ratios among the three measures (Table 1). In morphine pellet+morphine-injected mice, tolerance ranged from 25- to 36-fold with the same apparent rank order of tolerance development (Table 1). The magnitude of tolerance to hypothermia was greater than that which developed to Straub tail in the morphine pellet+morphine-treated group. These results demonstrate a similar relationship between morphine treatment paradigms and tolerance development in several tests of spinal and supraspinal actions of morphine, with the exception that slightly less tolerance developed to Straub tail induction than to other measures.
Table 1.
Comparison of tolerance levels to the supraspinally mediated effects of acutely administered s.c. morphine in mice chronically given a placebo pellet, a morphine pellet or a morphine pellet plus supplemental morphine injection
| Test | Placebo pellet ED50 mg kg−1 (95% CL) | Morphine pellet ED50 mg kg−1 (95% CL) | Potency ratio (95% CL) |
|---|---|---|---|
| Hot plate | 3.2 (2.4–4.3) | 23.1 (14.8–35.8)a | 7.3 (4.4–12.1)b |
| Hypothermiac | 3.4 (2.0–5.8) | 42.2 (18.4–96.6)a | 12.2 (6.6–27.1)b |
| % Straub tail | 3.5 (2.7–4.4) | 23.0 (20.3–26.1)a | 6.4 (5.1–8.2)b |
| |
+Saline |
+Morphine |
|
| Hot plate | 4.1 (3.5–4.9) | 144.5 (127.0–164.3)a | 35.4 (26.7–46.9)b |
| Hypothermiac | 4.6 (3.9–5.6) | 141.7 (103.2–194.7)a | 36.0 (29.3–44.3)b |
| % Straub tail | 4.7 (4.1–5.3) | 113.9 (99.8–130.0)a | 24.7 (21.1–28.9)b |
Abbreviations: CL, confidence limits; ED50, half-maximal effective dose; s.c., subcutaneously.
Mice were implanted with placebo pellet, 75 mg morphine pellet, placebo pellet+supplemental saline injection (+saline) or morphine pellet+supplemental morphine injection (+morphine), as described in Materials and methods. Thirty minutes after acute s.c. morphine treatment, the mice were assessed in the 56°C hot-plate test and for changes in body temperature (ΔTb) and Straub tail. Data are mean values±95% CL from 30 mice per group, including six mice per morphine dose (four doses each and saline).
Significantly different than placebo-P group based on non-overlapping 95% CLs.
Significantly different potency ratio because the lower 95% CL is greater than 1.
Calculated as the ED1.5 for a maximum ΔTb of −3°C.
Agonist-stimulated [35S]GTPγS autoradiography
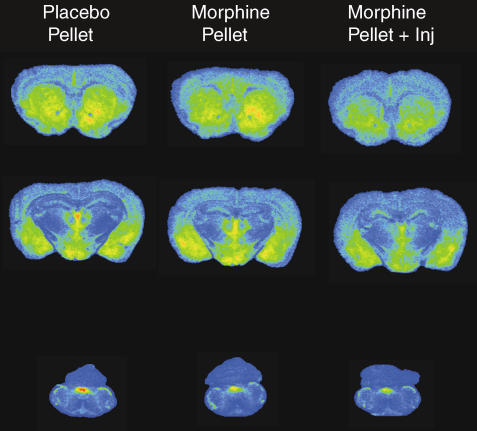
The anatomical distribution of DAMGO-stimulated [35S]GTPγS binding in brain and spinal cord of Swiss Webster mice corresponded to that previously described in rat (Sim et al., 1996). Representative brain sections from placebo- and morphine-treated mice are shown in Figure 2 and reveal decreased agonist-stimulated activity in certain regions of treated mice, particularly in brains from morphine pellet+morphine-treated animals. Densitometric analysis confirmed these observations and showed that DAMGO-stimulated [35S]GTPγS binding was significantly decreased in most regions examined from brains of morphine pellet+morphine-treated mice compared to placebo control, including nucleus accumbens, caudate-putamen, cingulate cortex, PAG, parabrachial nucleus, NTS and spinal cord dorsal horn (Table 2). In contrast, DAMGO-stimulated [35S]GTPγS binding was significantly reduced only in the NTS and spinal cord dorsal horn in morphine pellet-implanted compared to placebo pellet-implanted mice. A trend towards a decrease in DAMGO-stimulated [35S]GTPγS binding was found in PAG (−17%) of morphine pellet-implanted mice, but this value did not significantly differ from placebo pellet control. Examination of the data showed that regions with a significant decrease in MOR-mediated G-protein activation in morphine-pellet-implanted mice did not exhibit significant further loss in G-protein activation in morphine pellet+morphine-treated mice. A subset of regions also showed a significant loss in G-protein activity in morphine pellet+morphine-treated mice compared to mice treated with morphine pellet only (nucleus accumbens, caudate-putamen, hypothalamus and parabrachial nucleus). These results demonstrate a more regionally widespread loss of MOR-mediated G-protein activation in the CNS of morphine pellet+morphine-injected mice than those treated with morphine pellet implantation alone.
Figure 2.
DAMGO-stimulated [35S]GTPγS autoradiography in representative brain sections from mice implanted with placebo pellet, morphine pellet or morphine pellet+supplemental morphine injection. Sections were incubated with 0.04 nM [35S]GTPγS, 2 mM GDP and 10 μM DAMGO as described in Materials and methods. Areas examined densitometrically include: caudate-putamen, nucleus accumbens, cingulate cortex (row 1); thalamus, amygdala, hypothalamus (row 2); and NTS (row 3).
Table 2.
Net DAMGO-stimulated [35S]GTPγS autoradiography in specific CNS regions of mice administered placebo pellet (placebo-P), morphine pellet (morphine-P) or morphine pellet plus supplemental morphine injection (morphine-P+morphine)
| Region | Placebo-P |
Morphine-P |
Morphine-P+morphine |
||
|---|---|---|---|---|---|
| (nCi g−1) | (nCi g−1) | (% Cont.) | (nCi g−1) | (% Cont.) | |
| Nucleus accumbens | 256±23 | 237±13 | 92±5.0 | 153±13*† | 60±5.0 |
| Caudate-putamen | 201±17 | 191±16 | 95±7.9 | 120±10*† | 60±4.9 |
| Cingulate cortex | 132±8 | 110±4 | 83±3.0 | 89±8* | 67±6.0 |
| Thalamus | 188±20 | 183±22 | 97±11 | 130±21 | 64±11 |
| Amygdala | 230±9 | 210±17 | 91±7.3 | 197±15 | 86±6.5 |
| Hypothalamus | 143±13 | 180±12 | 110±7.3 | 123±11† | 80±5.5 |
| PAG | 88±7 | 73±14 | 83±15 | 47±7* | 53±7.9 |
| Parabrachial nucleus | 117±12 | 104±13 | 89±11 | 49±7*† | 42±5.9 |
| NTS | 236±24 | 168±23* | 71±9.7 | 157±10* | 67±4.2 |
| Spinal cord dorsal horn | 187±9 | 147±11* | 79±5.8 | 134±13* | 72±6.9 |
Abbreviations: CNS, central nervous system; DAMGO, [D-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin; GTPγS, guanylyl-5′-O-(γ-thio)-triphosphate; NTS, nucleus tractus solitarius; PAG, periaqueductal grey.
Mice were treated with placebo pellets, morphine pellets or morphine pellets+morphine and appropriate brain sections were processed for DAMGO-stimulated [35S]GTPγS autoradiography as described in Materials and methods. Data are mean net DAMGO-stimulated [35S]GTPγS binding±s.e. in nCi g−1 from brains from 10 mice per group.
P<0.05 different from control (placebo-P) mice by Dunnett's test;
P<0.05 different from mice with morphine pellets implanted only (morphine-P) by Newman–Keuls test.
Agonist-stimulated [35S]GTPγS binding in membranes
DAMGO-stimulated [35S]GTPγS binding was examined in concentration–effect curves in membranes from midbrain-dorsal pons, which contained PAG, and spinal cord of mice treated as described above. DAMGO-stimulated [35S]GTPγS binding increased in a concentration-dependent and saturable manner in both regions in all groups of mice (data not shown). Nonlinear regression analysis of the concentration–effect curves revealed that treatment with morphine pellets+morphine injection produced a significant decrease in the Emax value of DAMGO (100±7.6% in placebo pellet versus 78±3.9% in morphine pellet+morphine; P<0.05; n=6) in PAG, and a similar trend was observed in this region in morphine-pelleted mice (76±9.7%) but did not reach statistical significance. There was no difference in the DAMGO EC50 values, which ranged from 176 to 190 nM, among these groups. Basal [35S]GTPγS binding in this region was also not statistically different among groups. Similar results were obtained in spinal cord membranes (data not shown), although the apparent effect (Emax=87±7.3 and 78±6.3% of control in morphine pellet- or morphine pellet+morphine-treated mice, respectively) was below the level of statistical significance. Overall, these results are similar to those obtained in the [35S]GTPγS autoradiography experiments, suggesting that the apparent desensitization of MOR-mediated G-protein activation in sections from chronic morphine-treated mice was not an acute effect resulting from incubation of the sections with DAMGO.
MOR levels in membranes
To determine whether the decreases in MOR-mediated G-protein activation observed after chronic morphine treatment were due to decreased MOR levels, saturation binding analysis was conducted with [3H]naloxone in membranes prepared from several brain regions. Unlabeled DOR and KOR antagonists, naltrindole and norbinaltorphimine, were included in these experiments to ensure that naloxone was binding to MOR exclusively. Results showed that the Bmax values of MOR binding sites were not significantly decreased in morphine pellet- or morphine pellet+morphine-treated mice relative to control mice in any CNS region examined (Table 3). These results indicate that attenuated G-protein activation after chronic morphine treatment was not due to downregulation of MOR. Similarly, there was no difference in [3H]naloxone KD values between control mice and either of the morphine-treated groups in any region examined, suggesting that there was no residual morphine remaining in the tissue preparations.
Table 3.
[3H]Naloxone saturation analysis in membranes from CNS regions of mice, implanted with a placebo pellet (placebo-P), morphine pellet (morphine-P) or morphine pellet plus supplemental morphine injection (morphine-P+morphine)
|
Placebo pellet |
Morphine pellet |
Morphine pellet+morphine |
||||
|---|---|---|---|---|---|---|
| Region | Bmax (fmol mg−1) | KD (nM) | Bmax (fmol mg−1) | KD (nM) | Bmax (fmol mg−1) | KD (nM) |
| Striatum | 340±72 | 2.8±0.5 | 275±61 | 1.6±0.6 | 264±37 | 3.3±0.9 |
| Cingulate cortex | 203±26 | 3.0±0.8 | 256±38 | 4.5±1.2 | 220±78 | 2.3±0.8 |
| Thalamus | 297±53 | 1.6±0.3 | 357±16 | 2.9±0.7 | 311±55 | 2.2±0.5 |
| PAG | 348±50 | 1.8±0.4 | 383±49 | 1.6±0.5 | 527±130 | 3.0±0.7 |
| Medulla | 381±34 | 2.6±0.4 | 413±50 | 3.0±0.5 | 423±32 | 3.1±0.3 |
| Spinal cord | 355±50 | 2.7±0.4 | 384±85 | 2.7±0.5 | 441±89 | 3.3±0.4 |
Abbreviations: CNS, central nervous system; PAG, periaqueductal grey.
Membranes prepared from mice treated with placebo pellets, morphine pellets or morphine pellets+morphine were incubated with varying concentration of [3H]naloxone as described in Materials and methods. Data are mean Bmax and KD values±s.e. (n=4–6) from nonlinear regression analysis of saturation binding curves. Statistical significance was determined with Dunnett's test.
Discussion and conclusions
Chronic opioid treatment produces tolerance (Way et al., 1969), but a review of the literature suggests that various cellular mechanisms contribute depending on the opioid administration paradigm and behavioural measures. The present study used two chronic morphine paradigms to induce different levels of tolerance and evaluated MOR-mediated G-protein activity in multiple CNS regions in these mice. Our results showed that implantation of morphine pellet alone produced approximately 6- to 12-fold tolerance to morphine-mediated antinociception (tail withdrawal and hot plate), hypothermia and Straub tail, and that MOR-mediated G-protein activation was significantly attenuated in NTS and spinal cord. In contrast, injection of morphine in pellet-implanted mice produced approximately 25- to 50-fold tolerance and regionally widespread attenuation of MOR-mediated G-protein activity. Thus, dramatic differences were found in the regional pattern of chronic morphine-induced attenuation of MOR-mediated G-protein activation between mice rendered moderately versus highly tolerant to the in vivo effects of morphine. Neither group of mice exhibited downregulation of MOR binding sites, consistent with the hypothesis that this adaptation is not required for induction of tolerance to morphine. These results suggest a positive relationship between attenuation of MOR-mediated G-protein activation and tolerance that in most regions appears to be more associated with higher than lower levels of tolerance, suggesting the contribution of different mechanisms as tolerance progresses.
In vivo studies showed that tolerance develops to all behavioural measures to a similar degree, although slightly less tolerance was found for morphine-induced Straub tail. Administration of morphine pellets with supplemental morphine injections is a novel paradigm developed to increase the magnitude of tolerance (Smith et al., 2003). Presumably, increasing the morphine dose from 40 to 320 mg kg−1 day−1 was sufficient to induce a greater degree of morphine tolerance and more widespread attenuation of MOR-mediated G-protein activation by 72 h. Alternatively, it is possible that results differed between the treatment groups due to the intermittent nature of the supplemental injections because behavioural sensitization can occur after intermittent administration (Powell and Holtzman, 2001; Vigano et al., 2003). This is unlikely because intervening abstinence periods are generally required for sensitization (Powell and Holtzman, 2001; Vigano et al., 2003), and opioid-stimulated [35S]GTPγS binding was reportedly increased or unchanged in morphine-sensitized animals (Vigano et al., 2003). Similarly, the supplemental injections could have produced periods of relative withdrawal in the interval between injections. Opioid withdrawal has been associated with alterations in various cellular parameters such as elevations in cAMP and transcription factors (Nestler et al., 1994; McDaid et al., 2006), GABAA receptor expression (Jang et al., 2002) or perhaps most relevant to the present study, GRK2 expression (Ozaita et al., 1998; Fan et al., 2002). However, in the present study the release of morphine from pellets presumably continued during the time between injections. In fact, brain morphine levels are elevated up to 6 days after morphine pellet implantation in mice and morphine tolerance peaks at 3 days post-implantation (Patrick et al., 1975). These results suggest that significant withdrawal effects did not occur during the 72 h period of treatment in either the morphine pellet-implanted or morphine pellet+morphine-injected mice. This conclusion is supported by a lack of overt withdrawal signs seen during routine observation of these mice. Moreover, spontaneous or precipitated opioid withdrawal in rats reportedly did not alter MOR-stimulated [35S]GTPγS binding in several brain regions (Kirschke et al., 2002). Interestingly, morphine injection alone at regular intervals that produced tolerance was reported to attenuate MOR-stimulated [35S]GTPγS binding in brainstem of mice (Bohn et al., 2002) but not rats (Kirschke et al., 2002), suggesting possible species differences in the pharmacokinetic/pharmacodynamic relationship of MOR adaptation to chronic morphine.
Comparison of data from moderate (6- to 12-fold) versus high (25- to 50-fold) tolerant mice showed regional differences in apparent desensitization produced by the two paradigms, which are in agreement with our previous studies (Sim et al., 1996; Sim-Selley et al., 2000). A consistent finding has been that apparent desensitization of MOR-mediated G-protein activation or effector modulation occurs most readily in brainstem nuclei, such as PAG, parabrachial nuclei and NTS, whereas forebrain regions, such as caudate-putamen and nucleus accumbens, generally show a smaller magnitude of this effect (Noble and Cox, 1996; Sim et al., 1996; Sim-Selley et al., 2000). In the present study, morphine pellet implantation that induced moderate tolerance significantly reduced DAMGO-stimulated [35S]GTPγS binding only in NTS and spinal cord dorsal horn. In contrast, attenuation of DAMGO-stimulated [35S]GTPγS binding in brains from mice that received morphine pellet+morphine injections, that induced a higher level of tolerance, produced significant decreases in most CNS regions.
It is perhaps significant that all of the in vivo measures studied exhibited a similar baseline morphine potency (3–6 mg kg−1) and tolerance development after chronic treatment with either morphine pellet alone (6- to 12-fold) or morphine pellet+morphine injection (25- to 50-fold). The in vivo responses in the present study were primarily lower order CNS functions that are mainly thought to involve spinal, brainstem and hypothalamic circuits. However, studies of higher order behavioural responses such as drug discrimination or self-administration have generally found higher potencies of opioids and less tolerance development compared to antinociception (De Vry, 1989; Bergman, 2000). It will be of interest in future studies to compare the two chronic morphine treatment paradigms used in the present study in these higher order behaviours. We would predict that treatment with morphine pellet alone would not produce tolerance to discriminative stimulus or reinforcing effects of morphine, whereas the morphine pellet+morphine injection paradigm, which produced attenuation of MOR-mediated G-protein activation in forebrain areas, would produce tolerance to these effects.
Opioid-mediated antinociception involves activity in a number of CNS regions, notably the PAG, rostral ventral medulla and spinal cord dorsal horn (Fields and Basbaum, 1978). The anatomical circuitry involved is dependent on the analgesic test employed, and tail withdrawal is considered to be mainly a spinally mediated reflex. Both chronic treatment paradigms in this study produced attenuation of MOR-mediated G-protein activation in the spinal cord dorsal horn, which could contribute to the expression of both moderate and high levels of tolerance in the tail withdrawal test. In contrast, the hot-plate test is considered to have a greater supraspinal component, as do Straub tail and hypothermia, the latter of which is probably entirely supraspinal. For these measures, tolerance developed in the apparent absence of significant MOR adaptation in most brain regions (except NTS). There could be many explanations for these results. First, it is possible that desensitization of receptor-mediated G-protein activity is not necessary for tolerance development and that downstream adaptations, such as compensatory hyperalgesia, altered neuronal excitability and/or changes in gene expression mediate tolerance (Nestler et al., 1994; Ossipov et al., 2004). Alternatively, it is possible that analysis with current techniques is not sufficiently sensitive to detect modest changes in specific populations of MOR-containing neurons. It is important to consider that even in the autoradiographic assay it is not possible to isolate MOR-containing neurons that are part of specific neural circuits that contribute to behaviours of interest. For antinociception, it is also possible that attenuation of MOR signaling in spinal cord and medulla is sufficient for moderate tolerance, but a more regionally widespread loss in MOR signaling could be associated with greater tolerance by interfering with spinal/supraspinal synergy of opioid antinociception (Roerig et al., 1984). Another potential influence is adaptation in peripherally localized MORs that could contribute to antinociception, an effect that also exhibits to tolerance following chronic morphine treatment (Kolesnikov et al., 1996; Nozaki-Taguchi and Yaksh, 1999). Regardless of the explanation, these considerations reflect the complexity of defining opioid tolerance both mechanistically and anatomically.
The finding that most brain regions exhibited different levels of MOR adaptation following moderate versus high tolerance paradigms suggests that different mechanisms might be involved at various stages during the development of tolerance. For example, protein kinase C (PKC) inhibition reversed tolerance induced by morphine pellet implantation alone, whereas inhibition of PKC produced only a partial reversal of tolerance induced by morphine pellet+morphine injection (Smith et al., 2003). Inhibition of both PKA and PKC was required to completely reverse the higher level of tolerance, suggesting that overlapping but non-identical mechanisms could be involved in the development or maintenance of moderate versus high levels of morphine tolerance.
The attenuation of MOR-mediated G-protein activation in the present study was probably due to desensitization, or uncoupling of MOR from G-protein activation. This apparent desensitization of MOR-mediated signaling in brain has been found at the level of G-protein activation (Tao et al., 1993; Sim et al., 1996; Kruzich et al., 2003), and effector (Noble and Cox, 1996; Deng et al., 2001) after chronic opioid treatment. Similarly, tolerance to opioid-mediated increases in potassium conductance has been found in brain slices from chronic morphine-treated animals (Christie et al., 1987; Zhang et al., 1996). MOR desensitization probably results, at least in part, from phosphorylation of MOR followed by β-arrestin binding to phosphorylated receptors (Zhang et al., 1998; Deng et al., 2001). Studies in mice with targeted disruption of the β-arrestin-2 gene also suggest a role for this mechanism in opioid tolerance because the development of both antinociceptive tolerance and desensitization of brainstem MOR were decreased in mice lacking β-arrestin-2 (Bohn et al., 1999, 2000). Those results are consistent with our interpretation of the present findings indicating that attenuation of MOR-induced G-protein activation in specific CNS regions of mice chronically treated with morphine contributes to tolerance, especially after high-dose administration paradigms. However, the anatomically restricted nature of desensitization in mice that received the lower dose morphine paradigm did not correlate completely with in vivo tolerance, suggesting that other mechanisms are involved. These results are consistent with recent findings that multiple mechanisms contribute to opioid tolerance (Gintzler et al., 1997; Bailey et al., 2003) and underscore the complexity of mechanisms underlying opioid tolerance.
To our knowledge, this is the first study to vary the level of morphine tolerance, measured with multiple behaviours, and compare it with MOR desensitization and receptor levels throughout the CNS, in order to characterize neuroadaptation at both cellular and in vivo levels. These data reveal treatment-dependent differences in the regional distribution of apparent desensitization of MOR-mediated G-protein activation. These results suggest that differences in opioid administration regimens, particularly with respect to the dose of chronic treatment, could produce different profiles of neuroadaptation and tolerance.
Acknowledgments
This work was supported by USPHS Grants DA-01647, DA10770, DA-00480 and DA-07027 from the National Institute on Drug Abuse.
Abbreviations
- BSA
bovine serum albumin
- DAMGO
[D-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin
- GTPγS
guanylyl-5′-O-(γ-thio)-triphosphate
- MOR
μ opioid receptor
- NTS
nucleus tractus solitarius
- PAG
periaqueductal grey
Conflict of interest
The authors state no conflict of interest.
References
- Bailey CP, Couch D, Johnson E, Griffiths K, Kelley E, Henderson G. Opioid receptor desensitization in mature rat neurons: lack of interaction between DAMGO and morphine. J Neurosci. 2003;23:10515–10520. doi: 10.1523/JNEUROSCI.23-33-10515.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J, France CP, Holtzman SG, Katz JL, Koek W, Stephens DN. Agonist efficacy, drug dependence, and medications development: preclinical evaluation of opioid, dopaminergic, and GABAA-ergic ligands. Psychopharmacology (Berl) 2000;153:67–84. doi: 10.1007/s002130000567. [DOI] [PubMed] [Google Scholar]
- Bliss CI. Statistics in Biology. McGraw-Hill: New York; 1967. [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Caron MG. Differential mechanisms of morphine antinociceptive tolerance revealed in βarrestin-2 knock-out mice. J Neurosci. 2002;22:10494–10500. doi: 10.1523/JNEUROSCI.22-23-10494.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Selley DE, Childers SR. Acute and chronic effects of opioids on delta and mu receptor activation of G-proteins in NG108-15 and SK-N-SH cell membranes. J Neurochem. 1997;68:1462–1472. doi: 10.1046/j.1471-4159.1997.68041462.x. [DOI] [PubMed] [Google Scholar]
- Childers SR. Opioid receptor-coupled second messengers. Life Sci. 1991;48:1991–2003. doi: 10.1016/0024-3205(91)90154-4. [DOI] [PubMed] [Google Scholar]
- Christie MJ, Williams JT, North RA. Cellular mechanisms of opioid tolerance: studies in single brain neurons. Mol Pharmacol. 1987;32:633–638. [PubMed] [Google Scholar]
- Colquhoun D. Lectures on Biostatistics: An Introduction to Statistics with Applications in Biology and Medicine. Clarendon Press: Oxford; 1971. [Google Scholar]
- Deng HB, Yu Y, Wang H, Guang W, Wang JB. Agonist-induced μ opioid receptor phosphorylation and functional desensitization in rat thalamus. Brain Research. 2001;898:204–214. doi: 10.1016/s0006-8993(01)02179-5. [DOI] [PubMed] [Google Scholar]
- De Vry J, Donselaar I, Van Ree JM. Intraventricular self-administration of heroin in the rat: reward seems dissociated from analgesia and physical dependence. Eur J Pharmacol. 1989;161:19–25. doi: 10.1016/0014-2999(89)90175-1. [DOI] [PubMed] [Google Scholar]
- Fan X, Zhang J, Zhang X, Yue W, Ma L. Acute and chronic morphine treatments and morphine withdrawal differentially regulate GRK2 and GRK5 gene expression in rat brain. Neuropharmacology. 2002;43:809–816. doi: 10.1016/s0028-3908(02)00147-8. [DOI] [PubMed] [Google Scholar]
- Fields HL, Basbaum AI. Brainstem control of spinal pain-transmission neurons. Annu Rev Physiol. 1978;40:217–248. doi: 10.1146/annurev.ph.40.030178.001245. [DOI] [PubMed] [Google Scholar]
- Gintzler AR, Adapa ID, Toll L, Medina VM, Wang L. Modulation of enkephalin release by nociceptin (orphanin FQ) Eur J Pharmacol. 1997;325:29–34. doi: 10.1016/s0014-2999(97)00103-9. [DOI] [PubMed] [Google Scholar]
- Harris LS, Pierson AK. Some narcotic antagonists in the benzomorphan series. J Pharmacol Exp Ther. 1964;143:141–148. [PubMed] [Google Scholar]
- Jang SY, Kim Y, Oh S. The bindings of [3H]muscimol and [3H]flunitrazapam are elevated in discrete brain regions of butorphanol-withdrawal rats. Neurochem Res. 2002;27:939–946. doi: 10.1023/a:1020399716812. [DOI] [PubMed] [Google Scholar]
- Kameyama T, Nabeshima T, Ukai M, Yamaguchi K. Morphine-induced Straub tail reaction and spinal catecholamine metabolite content: antagonism of naloxone to morphine-induced effects in mice. Chem Pharm Bull (Tokyo) 1978;26:2615–2618. doi: 10.1248/cpb.26.2615. [DOI] [PubMed] [Google Scholar]
- Kirschke C, Schadrack J, Zieglgansberger W, Spanagel R. Effects of morphine withdrawal on μ-opioid receptor-stimulated guanylyl 5′-[γ[35S]thio]-triphosphate autoradiography in rat brain. Eur J Pharmacol. 2002;446:43–51. doi: 10.1016/s0014-2999(02)01763-6. [DOI] [PubMed] [Google Scholar]
- Kolesnikov YA, Jain S, Wilson R, Pasternak GW. Peripheral morphine analgesia: synergy with central sites and a target of morphine tolerance. J Pharmacol Exp Ther. 1996;279:502–506. [PubMed] [Google Scholar]
- Kruzich PJ, Chen ACH, Unterwald EM, Kreek MJ. Subject-regulated dosing alters morphine self-administration behavior and morphine-stimulated [35S]GTPγS binding. Synapse. 2003;47:243–249. doi: 10.1002/syn.10173. [DOI] [PubMed] [Google Scholar]
- Law PY, Wong YH, Loh HH. Molecular mechanisms and regulation of opioid receptor signaling. Annu Rev Pharmacol Toxicol. 2000;40:389–430. doi: 10.1146/annurev.pharmtox.40.1.389. [DOI] [PubMed] [Google Scholar]
- Loh HH, Liu HC, Cavalli A, Yang W, Chen YF, Wei LN. Mu opioid receptor knockout in mice: effects on ligand-induced analgesia and morphine lethality. Mol Brain Res. 1998;54:321–326. doi: 10.1016/s0169-328x(97)00353-7. [DOI] [PubMed] [Google Scholar]
- Maher CE, Eisenach JC, Pan H-L, Xiao R, Childers SR. Chronic intrathecal morphine administration produces homologous mu receptor/G-protein desensitization specifically in spinal cord. Brain Res. 2001;895:1–8. doi: 10.1016/s0006-8993(00)03093-6. [DOI] [PubMed] [Google Scholar]
- Matthes HWD, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the μ-opioid receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- McDaid J, Dallimore JE, Mackie AR, Napier TC. Changes in accumbal and pallidal pCREB and deltaFosB in morphine-sensitized rats: correlations with receptor-evoked electrophysiological measures in the ventral pallidum. Neuropsychopharmacology. 2006;31:1212–1226. doi: 10.1038/sj.npp.1300854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Alreja M, Aghajanian GK. Molecular and cellular mechanisms of opiate action: studies in the locus coeruleus. Brain Res Bull. 1994;35:521–528. doi: 10.1016/0361-9230(94)90166-x. [DOI] [PubMed] [Google Scholar]
- Noble F, Cox BM. Differential desensitization of mu- and delta-opioid receptors in selected neural pathways following chronic morphine treatment. Br J Pharmacol. 1996;117:161–169. doi: 10.1111/j.1476-5381.1996.tb15169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki-Taguchi N, Yaksh TL. Characterization of the antihyperalgesic action of a novel peripheral mu-opioid receptor agonist – loperamide. Anesthesiology. 1999;90:225–234. doi: 10.1097/00000542-199901000-00029. [DOI] [PubMed] [Google Scholar]
- O'Callaghan JP, Holtzman SG. Quantification of the analgesic activity of narcotic antagonists by a modified hot-plate procedure. J Pharmacol Exp Ther. 1975;192:497–505. [PubMed] [Google Scholar]
- Ossipov MH, Lai J, King T, Vanderah TW, Malan TP, Jr, Hruby VJ, et al. Antinociceptive and nociceptive actions of opioids. J Neurobiol. 2004;61:126–148. doi: 10.1002/neu.20091. [DOI] [PubMed] [Google Scholar]
- Ozaita A, Escriba PV, Ventayol P, Murga C, Mayor JF, Garcia-Sevilla JA. Regulation of G protein-coupled receptor kinase 2 in brains of opiate-treated rats and human opiate addicts. J Neurochem. 1998;70:1249–1257. doi: 10.1046/j.1471-4159.1998.70031249.x. [DOI] [PubMed] [Google Scholar]
- Patel MB, Patel CN, Rajashekara V, Yoburn BC. Opioid agonists differentially regulate μ-opioid receptors and trafficking proteins in vivo. Mol Pharmacol. 2002;62:1464–1470. doi: 10.1124/mol.62.6.1464. [DOI] [PubMed] [Google Scholar]
- Patrick GA, Dewey WL, Spaulding TC, Harris LS. Relationship of brain morphine levels to analgesic activity in acutely treated mice and rats and in pellet implanted mice. J Pharmacol Exp Ther. 1975;193:876–883. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press: San Diego, CA; 1986. [Google Scholar]
- Powell KR, Holtzman SG. Parametric evaluation of the development of sensitization to the effects of morphine on locomotor activity. Drug Alcohol Depend. 2001;62:83–90. doi: 10.1016/s0376-8716(00)00167-8. [DOI] [PubMed] [Google Scholar]
- Puttfarcken PS, Cox BM. Morphine-induced desensitization and downregulation at mu-receptors in 7315C pituitary tumor cells. Life Sci. 1989;45:1937–1942. doi: 10.1016/0024-3205(89)90548-1. [DOI] [PubMed] [Google Scholar]
- Roerig SC, O'Brien SM, Fujimoto JM, Wilcox GL. Tolerance to morphine analgesia: decreased multiplicative interaction between spinal and supraspinal sites. Brain Res. 1984;308:360–363. doi: 10.1016/0006-8993(84)91078-3. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Childers SR. In vitro autoradiography of receptor-activated G-proteins in rat brain by agonist-stimulated guanylyl 5′-[γ-[35S]thio]-triphosphate binding. Proc Natl Acad Sci USA. 1995;92:7242–7246. doi: 10.1073/pnas.92.16.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Dworkin SI, Childers SR. Effects of chronic morphine administration on mu opioid receptor-stimulated [35S]GTPγS autoradiography in rat brain. J Neurosci. 1996;16:2684–2692. doi: 10.1523/JNEUROSCI.16-08-02684.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ, Selley DE, Vogt LJ, Childers SR, Martin TJ. Chronic heroin self-administration desensitizes mu opioid receptor-activated G-proteins in specific regions of rat brain. J Neurosci. 2000;20:4555–4562. doi: 10.1523/JNEUROSCI.20-12-04555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FL, Javed RR, Elzey MJ, Dewey WL. The expression of a high level of morphine antinociceptive tolerance in mice involves both PKC and PKA. Brain Res. 2003;985:78–88. doi: 10.1016/s0006-8993(03)03170-6. [DOI] [PubMed] [Google Scholar]
- Stafford K, Gomes AB, Shen J, Yoburn BC. Opioid receptor downregulation contributes to opioid tolerance in vivo. Pharmacol Biochem Behav. 2001;69:233–237. doi: 10.1016/s0091-3057(01)00525-1. [DOI] [PubMed] [Google Scholar]
- Tao P-L, Law P-Y, Loh HH. Decrease in delta and mu opioid receptor binding capacity in rat brain after chronic etorphine treatment. J Pharmacol Exp Ther. 1987;240:809–816. [PubMed] [Google Scholar]
- Tao P-L, Lee C-R, Law P-Y, Loh HH. The interaction of the mu-opioid receptor and G protein is altered after chronic morphine treatment in rats. Nauyn-Schmiedeberg's Arch Pharmacol. 1993;348:504–508. doi: 10.1007/BF00173210. [DOI] [PubMed] [Google Scholar]
- Tian M, Broxmeyer HE, Fan Y, Lai Z, Zhang S, Aronica S, et al. Altered hematopoiesis, behavior, and sexual function in μ opioid receptor-deficient mice. J Exp Med. 1997;185:1517–1522. doi: 10.1084/jem.185.8.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigano D, Rubino T, Di Chiara G, Ascari I, Massi P, Parolaro D. Mu opioid receptor signaling in morphine sensitization. Neuroscience. 2003;117:921–929. doi: 10.1016/s0306-4522(02)00825-4. [DOI] [PubMed] [Google Scholar]
- Way EL, Loh HH, Shen FH. Simultaneous quantitative assessment of morphine tolerance and physical dependence. J Pharmacol Exp Ther. 1969;167:1–8. [PubMed] [Google Scholar]
- Zhang G, Lagrange AH, Ronnekleiv OK, Kelly MJ. Tolerance of hypothalamic β-endorphin neurons to μ-opioid receptor activation after chronic morphine. J Pharmacol Exp Ther. 1996;277:551–558. [PubMed] [Google Scholar]
- Zhang J, Ferguson SSG, Barak LS, Bodduluri SR, Laporte SA, Law P-Y, et al. Role for G protein-coupled receptor kinase in agonist-specific regulation of μ-opioid receptor responsiveness. Proc Natl Acad Sci USA. 1998;95:7157–7162. doi: 10.1073/pnas.95.12.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]