Abstract
Background and purpose:
Although CB1 receptor activation evokes neuroprotection in response to cannabinoids, some cannabinoids have been reported to be peroxisome proliferator activated receptor (PPAR) ligands, offering an alternative protective mechanism. We have, therefore, investigated the ability of a range of cannabinoids to activate PPARα and for N-oleoylethanolamine (OEA), an endogenous cannabinoid-like compound (ECL), to evoke neuroprotection.
Experimental approach:
Assays of PPARα occupancy and gene transactivation potential were conducted in cell-free and transfected HeLa cell preparations, respectively. In vivo estimates of PPARα activation through fat mobilization and gene transcription were conducted in mice. Neuroprotection in vivo was investigated in wild-type and PPARα gene-disrupted mice.
Key results:
The ECLs OEA, anandamide, noladin ether and virodhamine were found to bind to the purified PPARα ligand binding domain and to increase PPARα-driven transcriptional activity. The high affinity synthetic CB1/2 cannabinoid agonist WIN 55212-2 bound to PPARα equipotently with the PPARα agonist fenofibrate, and stimulated PPARα-mediated gene transcription. The phytocannabinoid Δ9 tetrahydrocannabinol was without effect. OEA and WIN 55212-2 induced lipolysis in vivo, while OEA pre-treatment reduced infarct volume from middle cerebral artery occlusion in wild-type, but not in PPARα-null mice. OEA treatment also led to increased expression of the NFκB-inhibitory protein, IκB, in mouse cerebral cortex, while expression of the NFκB-regulated protein COX-2 was inhibited.
Conclusions and implications:
These data demonstrate the potential for a range of cannabinoid compounds, of diverse structures, to activate PPARα and suggest that at least some of the neuroprotective properties of these agents could be mediated by nuclear receptor activation.
Keywords: cannabinoids, N-oleoylethanolamine, peroxisome proliferator-activated receptors (PPARs), neuroprotection
Introduction
The cannabinoids are a structurally diverse family of compounds with a large number of different biological targets. Many of their effects are mediated by CB1 and CB2 receptors, two 7-transmembrane G protein-coupled receptors that have been cloned and well characterized (Howlett, 2002; Di Marzo et al., 2004; Mackie, 2006), although several studies have produced evidence supporting the existence of other non-CB1/non-CB2 G protein-coupled receptors (Jarai et al., 1999; Breivogel et al., 2001; Hajos et al., 2001), possibly including the orphan GPR55 (Baker et al., 2006). Cannabinoids have been reported to stimulate other non-G protein-coupled receptors, the best characterized of these being the transient receptor potential vanilloid receptor 1 (TRPV1) or vanilloid receptor channel, which is activated by endogenous cannabinoids such as anandamide. Indeed, TRPV1 has been proposed to be the ionotropic cannabinoid receptor (Di Marzo et al., 2002).
In addition to these cell-surface cannabinoid receptors, there is growing evidence that the intracellular peroxisome proliferator-activated receptors (PPARs) are cannabinoid targets. The PPARs function as lipid-sensing receptors and, through the activation or repression of large sets of particular genes, they are intimately involved in the regulation of crucial metabolic events (Berger et al., 2005). The endogenous cannabinoid system is also involved in the control of energy balance as demonstrated by reductions in calorie intake, total fat mass and body weight in CB1-null mice (Cota et al., 2003b). PPARγ has recently been proposed as a cannabinoid target (Burstein, 2005) and we have also recently demonstrated that some of the longer term vascular effects of the plant cannabinoid tetrahydrocannabinol (Δ9THC) are mediated by this entity (O'Sullivan et al., 2005). Some effects of endogenous cannabinoid-like molecules (ECLs) appear to be due to activation of other PPARs; regulation of appetite and lipolysis by N-oleoylethanolamine (OEA) and the anti-inflammatory effects of N-palmitoylethanolamine have been demonstrated to occur via interaction with PPARα (Fu et al., 2003; Guzman et al., 2004; Lo Verme et al., 2005).
In this study, we have sought to determine whether cannabinoids with radically distinct structures interact with PPARα by assessing ligand binding and receptor activation in vitro. Since the selective PPARα ligand fenofibrate has been shown to provide neuroprotection in a mouse model of cerebral ischaemia (Deplanque et al., 2003), we have also examined the potential neuroprotective effects of the endogenous cannabinoid/PPARα agonist OEA in vivo.
The results presented demonstrate that a number of cannabinoid compounds are functionally effective PPARα agonists and that OEA has neuroprotective properties via PPARα activation. This indicates an additional mechanism by which the diverse effects of this physiologically and therapeutically important group of agents are mediated.
Methods
In vitro studies
Purification of the mPPARα–LBD–GST fusion protein
A mouse PPARα cDNA fragment was amplified from cDNA generated from mouse liver mRNA by reverse transcription (upper primer: 5′-CTGCCTTCCCTGTGAACTGACGTTTGTGGC-3′; lower primer: 5′-TGTGCAAATCCCTGCTCTCCTGTATGGGGC-3′). The cDNA encoding the mouse PPARα ligand-binding domain (LBD) with a deletion of the N-terminal A/B domain and mouse PPARα DNA-binding domain (amino acids 198–468) were ligated into pGEX-4T-1, a glutathione S-transferase (GST)-tagged bacterial expression vector, to generate the plasmid pGEX-mPPAR-LBD. The sequence-confirmed plasmid was transformed into Escherichia coli strain XL-10 and bacteria containing pGEX-mPPAR-LBD were cultured to OD600 0.6–0.8 at 37 °C. Expression of the GST-tagged LBD was induced with 0.2 mM isopropyl β-D-1-thiogalactopyranoside at 28 °C for 5 h. Lysates from these cultures were prepared and the expressed fusion protein was purified by glutathione-Sepharose 4B (Amersham Pharmacia, Chalfont St Giles, UK) with an elution buffer containing 20 mM reduced glutathione and 0.25% 3-(3-cholamidopropyl)dimethylammonio]-propanesulphonic acid in Tris-buffered saline buffer (10 mM Tris, 150 mM NaCl, pH 7.8). The purity of the resulting protein was assessed by sodium dodecyl sulphate-polyacrylamide gel electrophoresis followed by Coomassie blue staining and by immunoblotting using an anti-PPARα antibody (Biomol, Exeter, UK). Protein concentrations were determined by the Coomassie blue method (BioRad, Hemel Hempsted, UK).
Binding of cis-parinaric acid
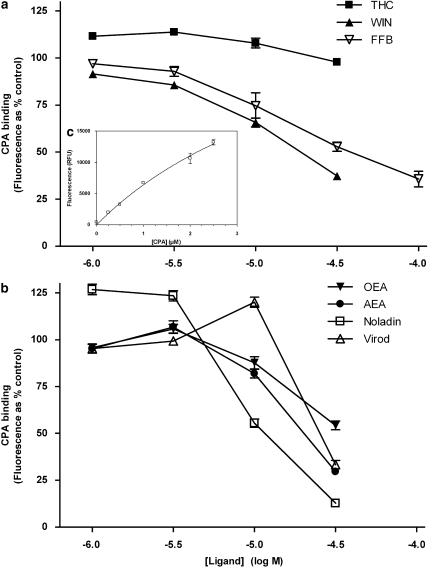
Binding of cis-parinaric acid (CPA) to the mPPARα LBD was monitored by measuring the enhanced fluorescence of the probe in the presence of the LBD protein (Causevic et al., 1999). Purified protein was diluted to 1 μM in Tris-buffered saline buffer; diluted proteins were combined with increasing quantities of CPA from a concentrated solution (3 mM) in ethanol. Protein and probe mixtures, 330 μl per well, were loaded into 96-well solid black-walled microplates. Fluorescence output (excitation, 315 nm; emission, 415 nm) was measured by reading the plate from the top using a FlexStation II plate reader (Molecular Devices, Sunnyvale, California, USA) at 25 °C. Background fluorescence, due to CPA in the absence of protein, was subtracted from each data point. The apparent Kd value for CPA was calculated from LBD saturation curves using GraphPad Prism (Figure 1). The affinities of non-fluorescent ligands for the LBD were calculated as IC50 values (concentrations reducing CPA/LBD fluorescence by 50%) from competition curves. Increasing concentrations of ligand were incubated in the presence of 1 μM mPPARα LBD and 2 μM CPA. Fluorescence was measured as above and corrected for background (drugs and CPA only).
Figure 1.
Binding of cis-parinaric acid (CPA) to mPPARα (GST fusion mouse PPARα ligand-binding domain). Concentration-dependent inhibition of CPA (2 μM) binding to mPPARα (1 μM) in the presence of selected synthetic (a) and endogenous (b) compounds. The inset c shows the concentration dependence of specific CPA binding (fluorescence in the presence of mPPARα−the fluorescence in the absence of mPPARα). Data are means±s.e.mean of three experiments conducted in quadruplicate and are expressed as % CPA-derived fluorescence in the absence of competing ligand (a and b) or as relative fluorescence units (c). PPAR, peroxisome proliferator-activated receptor.
Reporter gene activity of PPARα
Full-length mouse PPARα, including both DNA-binding domain and LBD, was ligated into pcDNA3.1/Zeo+ to generate the plasmid pcDNAwmPPAR. HeLa cells were maintained in Dulbecco's modified Eagle's medium with 10% heat-inactivated fetal bovine serum, 2 mM L-glutamine, 50 μg ml−1 penicillin and 50 μg ml−1 streptomycin at 37 °C, 5% CO2. HeLa cells, 60–70% confluent, in six-well plates were transiently transfected with pcDNAwmPPAR (0.5 μg) together with the reporter plasmid PPRE (1 μg per well). The reporter contains three PPAR-binding sites linked to a promoter controlling the gene for firefly luciferase. Transient transfection was carried out by the polyethyleneimine method with the ratio nitrogen (N) to DNA phosphate (P) of N/P=15 (Boussif et al., 1995; Turk et al., 2004). Immediately before transfection, cells were rinsed with phosphate-buffered saline (PBS) and supplemented with fresh serum-free culture medium. The plasmid DNA and the desired amount of polyethyleneimine polymer solution were each diluted in 150 mM NaCl and vortexed. The two solutions were then mixed and vortexed. After 20 min, the transfection mixture was added to the cells. After 4 h incubation, the medium was supplemented with 10% heat-inactivated fetal bovine serum; 16 h later, the complete medium was replaced with new Dulbecco's modified Eagle's medium with 0.5% heat-inactivated fetal bovine serum. Then 12 h later, appropriate amounts of drugs were added and cells were collected after another 15–16 h. Luciferase gene expression was monitored by luminometry using a commercial kit (Promega, Southampton, UK). Each transfection experiment was done in sextuplicate and results expressed as means±s.e.mean of relative luciferase activities normalized by cell protein concentration. Each experiment was repeated several times; absolute values varied sometimes within an order of magnitude depending on plasmid batch and the history of the cells, whereas relative values stayed stable.
Immunoblotting
Wild-type mice (C57BL/6) were treated for 3 days with OEA (10 mg kg−1 intraperitoneally (i.p.)) and killed 1 h after the last dose. The brains were rapidly dissected and cerebral cortex tissues were frozen at −80°C. On the day of use, tissues were homogenized in lysis buffer (0.5% v v−1 Nonidet P40, 0.1% w v−1 sodium deoxycholate, 0.001% w v−1 sodium dodecyl sulphate in PBS) before being centrifuged at 13 000 g for 10 min. The supernatant layers were standardized for protein content (50 μg protein per sample), separated on 10% sodium dodecyl sulphate gels at 100 mA and transferred onto nitrocellulose membranes (Hybond ECL, Amersham Pharmacia, Chalfont St Giles, UK) by electrophoresis at 200 mA for 1 h. The membranes were incubated in 5% w v−1 skimmed milk powder in PBS Tween (0.1% v v−1) for 1 h at room temperature, followed by a 60 min incubation at room temperature, or overnight at 4 °C, with primary antibody in 5% w v−1 skimmed milk powder, PBS Tween (0.1% v v−1). Membranes were rinsed rapidly three times, followed by a further three 20 min washes in PBS Tween, before incubation for 1 h with the appropriate secondary antibody in 1% w v−1 skimmed milk powder, PBS Tween. After being rinsed three times (20 min washes in PBS Tween), membranes were developed using the enhanced chemiluminescence western blotting detection system (Amersham Pharmacia, Chalfont St Giles, UK) according to the manufacturer's protocol. Immunoblots were scanned and digitized images were quantified by densitometry using AIDA 2.0 software; levels of immunoreactivity were compared with actin immunoreactivity from the same samples.
In vivo studies
Mouse middle cerebral artery occlusion
Male mice (wild-type C57BL/6 or PPARα knockouts C57BL/6 PPARα−/−, the latter obtained from Jackson Labs, Bar Harbor Maine, USA) were treated for 3 consecutive days with drugs (10 mg kg−1 per day i.p.). Twenty four hours later, cerebral ischaemia was induced by occlusion of the right middle cerebral artery as described previously (Gibson and Murphy, 2004). Following 60 min of middle cerebral artery occlusion (MCAO), mice were anaesthetized with 4% isoflurane (in an NO2/O2 70/30 mixture) and maintained by inhalation of 1.5% isoflurane. The occluding filament was withdrawn gently back into the common carotid artery to allow reperfusion to take place. In one experiment, mice were given a single injection of OEA at the end of the occlusion period. Final lesion volume was determined histologically 48 h later. Mice were killed by an overdose of pentobarbitone (i.p.) and the brains were sectioned into five 2-mm coronal slices using a mouse brain matrix (ASI Instruments, Warren, MI, USA). To quantify ischaemic damage, coronal slices were stained with 2% w v−1 2,3,5-triphenyltetrazolium chloride in saline for 30 min at room temperature in the dark. The area of infarction was measured on the posterior surface of each coronal section using a BioQuant IV image analysis system (Bioquant Inc., Ann Arbor, MI, USA). Because of substantial hemispheric swelling following ischaemia, infarct areas were calculated by a subtractive method in which the overestimation of infarct area due to the contribution of oedema is avoided. The infarcted area of the right (ischaemic) hemisphere was determined by subtracting the non-infarcted area of the right hemisphere from the total area of the left (uninfarcted) hemisphere. Total infarct volume was then determined by multiplying the area of infarct for each slice by the slice thickness (2 mm) and summing for all slices.
Stimulation of lipolysis
Lipolysis in vivo was assessed following injection of mice with 10 mg kg−1 (i.p.) test compound. Drugs were dissolved in dimethylsulphoxide (DMSO) to 10 mM, then diluted in normal saline to the appropriate concentration. The injection volume was 300 μl per mouse, with no animal receiving in excess of 25 μl DMSO. Blood was collected 1 h later in lithium heparin tubes (BD Biosciences, Oxford, UK), adding ethylene glycol tetraacetic acid to 1 mM and then centrifuging carefully at 2500 g for 20 min. Plasma was carefully transferred into new Eppendorf tubes and blood plasma-free fatty acids (FFA) were measured with a commercial kit (Wako Chemicals, Richmond, Vancouver, USA, 994–75409).
Animals
This study was conducted in accordance with the UK Animals (Scientific Procedures) Act, 1986 (Project Licences 40/2206 and PP 40/2715). All mice were housed in a pathogen-free facility at the University of Nottingham with access to food and water ad libitum. PPARα-null mice were expressed on a C57 background, while wild-type mice (C57, male, 20–35 g) were bred at the Biomedical Services Unit, University of Nottingham. Animals were group-housed (3–4 per cage) and lighting was provided from 0700 to 1900 hours, with all procedures carried out between 0800 and 1700 hours.
Materials
Plasmids and antibodies
The plasmids pGEM-T Easy, pGEX-4T-1 and pcDNA3.1/Zeo+ were purchased from Promega, Amersham Pharmacia and Invitrogen, Paisley, UK, respectively. Antibodies directed against actin, pan-PPAR, COX-2 (human) and IκBα (mouse) were purchased from Sigma, Gillingham, UK, Biomol, Cayman, Cayman Europe, Tallinn, Estonia, and Cell Signalling Technologies, Danvers, Massachusetts, USA, respectively.
Chemicals
All chemicals were purchased from Sigma (Poole, UK) or BDH Laboratory, Poole, UK, Supplies, unless otherwise stated. Anandamide, noladin ether, virodhamine and WIN 55212-2 ((R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo-[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate) were purchased from Tocris (Avonmouth, UK). OEA was synthesized (SPHA) in the School of Chemistry, University of Nottingham.
Results
In vitro binding to the mPPARα ligand-binding domain
cis-Parinaric acid bound to the GST-mouse PPARα-LBD fusion protein in a saturable fashion with a calculated Kd value of 5.4 μM (Figure 1c). Representatives of each of the major structural cannabinoid groups were assessed for their ability to bind to the LBD. The synthetic cannabinoid, WIN 55212-2, decreased the fluorescence of CPA in the presence of PPARα LBD in a concentration-dependent fashion with an apparent affinity slightly higher than that of the standard PPARα agonist fenofibrate (20 and 40 μM, respectively), while, the phytocannabinoid Δ9THC failed to alter CPA binding to PPARα LBD at concentrations up to 30 μM (Figure 1a). OEA has been shown to be a PPARα ligand (Fu et al., 2003) and it was found to decrease CPA-evoked fluorescence with an affinity between that of WIN 55212-2 and fenofibrate (EC50 value ∼30 μM). The putative endocannabinoids, anandamide, noladin ether and virodhamine all appeared to bind to PPARα with broadly similar affinities having a rank order of noladin ether>anandamide>virodhamine (EC50 values 10–30 μM, Figure 1b).
Activation of the mPPARα in vitro
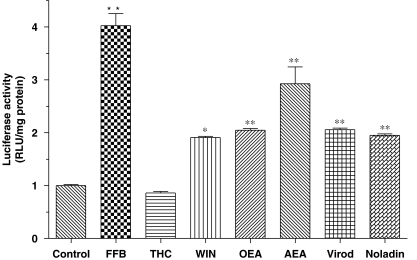
Although the high-affinity CB1/CB2 receptor agonist, WIN 55212-2 showed similar affinity at the PPARα LBD compared to the PPARα ligand fenofibrate (Figure 1a), its efficacy with regard to PPARα transcriptional activity was apparently much lower (Figure 2), although its maximum effect might have been compromised by toxicity at concentrations higher than 1 μM (data not shown). As expected, the phytocannabinoid, Δ9THC, which did not bind to the PPARα LBD in the CPA displacement assay (Figure 1a), failed to alter PPARα transcriptional activity (Figure 2). At 10 μM, the fatty acid ethanolamides, OEA and anandamide both evoked significant increases in luciferase gene expression (Figure 2). However, in a similar manner to WIN 55212-2, anandamide reduced the viability of HeLa cells at a high concentration (>10 μM) in our transient transfection system as measured by total protein concentrations (data not shown). Although they have been studied less than anandamide, noladin ether and virodhamine are putative endocannabinoids and both of them were found to double the PPARα-driven transcription at 10 μM (Figure 2). Although structurally similar to anandamide, their toxicity for HeLa cells was much less as measured by total protein concentrations (data not shown). Investigation of the effects of WIN 55212-2 and anandamide in reporter gene assays using other host cells (HEK293 human embryonic kidney and Chinese hamster ovary fibroblast cells) indicated a much greater tolerance, indicating that these effects on cell viability are not ubiquitous (data not shown).
Figure 2.
The effects of selected synthetic and endogenous compounds on PPARα-mediated transcriptional activity. HeLa cells were transiently co-transfected with PPARα and the PPRE-luc reporter gene constructs. Thereafter the cells were exposed to ligands overnight and the generation of luciferase estimated using a commercial luminometry kit. With the exception of WIN 55212-2 (WIN, 1 μM), ligands were present at 10 μM. Data are means±s.e.mean of three experiments conducted in sextuplicate. *P<0.05, **P<0.01 vs control, one-way analysis of variance with post hoc Dunnett's multiple comparison test. PPARα, peroxisome proliferator-activated receptor-α.
Cannabinoid effects in vivo
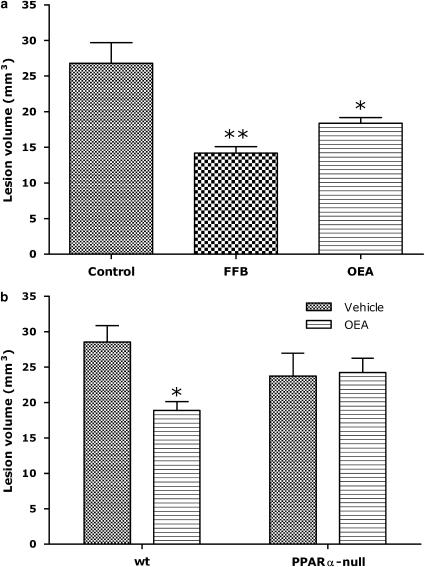
Intraperitoneal administration of OEA as a solution (8% v v−1 DMSO) evoked no visible behavioural effects in treated mice. In the mouse MCAO model of cerebral ischaemia, a single 1-h treatment with OEA at the end of the occlusion period had no effect on lesion size (data not shown). However, both fenofibrate and OEA treatments were found to reduce infarct volume significantly following 3 days of pre-treatment (Figure 3a). For example, lesion volume in untreated animals was 27±3 mm3, compared to 18±1 mm3 following OEA administration. This protective effect appeared to be PPARα-mediated since it was not apparent in the PPARα-null mice (Figure 3b). Lesion volumes in the vehicle-treated knockout animals were also not significantly different from the wild-type controls (Figure 3b).
Figure 3.
Effects of ligands on lesion volume in the mouse middle cerebral artery occlusion model in wild-type (a) and PPARα-null (b) mice. (a) Wild-type C57 mice were exposed to 60 min occlusion following three daily injections of OEA (10 mg kg−1, n=4), fenofibrate (10 mg kg−1, n=4) or vehicle (0.1 ml 10% DMSO, n=5). (b) PPAR-null or wild-type littermate C57 mice were exposed to 60 min occlusion following three daily injections of OEA (10 mg kg−1 day−1, n=5 and 4 for wt and PPARα-null, respectively) or vehicle (0.1 ml 10% DMSO, n=6 and 4 for wt and PPARα-null, respectively). *P<0.05, **P<0.01 vs vehicle treatment, one-way analysis of variance with Dunnett's multiple comparison (a) or two-way analysis of variance with Bonferroni comparison (b). DMSO, dimethylsulphoxide; OEA, N-oleoylethanolamine; PPARα, peroxisome proliferator-activated receptor-α; wt, wild-type.
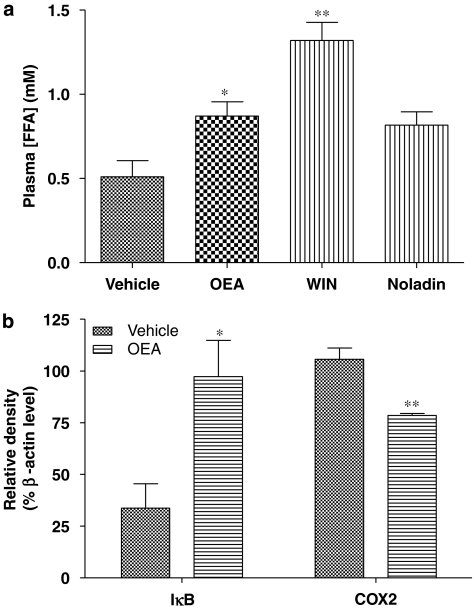
OEA and WIN 55212-2 (at 10 mg kg−1) were found to increase significantly plasma FFA levels after 1 h of exposure (Figure 4a). The putative endocannabinoid noladin ether, which showed increased affinity in binding to the PPARα in vitro compared to OEA (Figure 1b), although with comparable efficacy (Figure 2), also appeared to increase lipolysis in wild-type mice, but this effect failed to reach statistical significance (Figure 4a).
Figure 4.
Responses to ECLs in wild-type C57bl mice in vivo. (a) Lipolysis, as assessed by plasma-free fatty acid (FFA) levels, was determined 60 min following a single 10 mg kg−1 i.p. injection of the ECL. Data are means±s.e.mean from four (vehicle and noladin) or five (OEA and WIN 55212-2) determinations. *P<0.05, **P<0.01 vs vehicle treatment, one-way analysis of variance with Dunnett's multiple comparison test. (b) Influence of repeated injection of OEA (3 days at 10 mg kg−1 per day i.p.) administration on levels of inflammatory modulator expression in the mouse cerebral cortex. Data are means±s.e.mean of three determinations of immunoreactivity expressed as a percentage of actin immunoreactivity from the same samples. *P<0.05, **P<0.01 vs vehicle treatment, by Student's t-test. ECL, endogenous cannabinoid-like compound; OEA, N-oleoylethanolamine.
Since PPARα pre-activation by OEA was found to be neuroprotective, the involvement of two PPARα-regulated genes known to be involved in the control of inflammation, were investigated in the mouse cerebral cortex. The NF-κB pathway, which plays an important role in the inflammatory response, was assessed by measuring expression of its inhibitory protein IκBα by immunoblotting. The cerebral cortices of OEA-pre-treated mice were found to express almost three times the levels of IκBα compared to tissue from vehicle-treated animals (Figure 4b). The expression of the inflammatory marker enzyme COX-2 was also found to be reduced significantly in the cerebral cortex after OEA pre-treatment (Figure 4b).
Discussion
In this study, we describe a novel mechanism for cannabinoid-induced neuroprotection in vivo, via activation of the nuclear receptor, PPARα. Repeated dosing with the putative endocannabinoid OEA increased central nervous system (CNS) levels of the anti-inflammatory mediator IκBα, while decreasing the pro-inflammatory enzyme COX-2, and also reduced infarct volume following MCAO in a PPARα-dependent manner.
Functional targets of endocannabinoids
The term cannabinoid describes a structurally diverse group of compounds that can potentially bind to (at least) two 7-transmembrane cannabinoid receptors (CB1 and CB2). Although many of the physiological responses to cannabinoids, such as alterations in cognition and memory, euphoria, immobility, analgesia, hypothermia and sedation (Howlett, 1995), are generally thought to be due to CB receptors, studies with CB1, CB2 or CB1/CB2 double-knockout mice have revealed some non-CB receptor-mediated responses to cannabinoids both in the CNS and periphery (Howlett et al., 2002). Although perhaps not a direct agonist of cannabinoid receptors (Lambert et al., 1999), being a structural analogue of anandamide, OEA has cannabimimetic effects by competing with anandamide for the endocannabinoid-metabolizing enzyme, fatty acid amide hydrolase (Jonsson et al., 2001) and can be considered an ECL. OEA has recently been shown to be the endogenous ligand of an ‘orphan' receptor GPR119, which appears to be localized primarily to gut-associated organs, with some CNS expression (Overton et al., 2006). OEA activity at the nuclear receptor PPARα has also been demonstrated recently; OEA was observed to regulate feeding behaviour and body weight and to induce lipolysis via PPARα-dependent mechanisms (Fu et al., 2003; Guzman et al., 2004). Because of the possibility that other cannabinoids might share this last property with OEA, the main aim of the present study was to test the affinity and efficacy of different cannabinoids at PPARα and to investigate physiological consequences of in vivo exposure to such agents.
PPARα activity of synthetic and endogenous cannabinoids
By the use of multiple in vitro and in vivo assays, OEA was confirmed as a bona fide PPARα ligand. Indeed, in the in-vitro binding assay, OEA showed a similar potency to fenofibrate (Figure 1), a classical PPARα agonist, although it lacked the efficacy of fenofibrate in the PPARα reporter gene assay (Figure 2). Furthermore, assessment of the in vivo effects of OEA indicated that the protective effects of three daily-repeated doses on infarct volume in a mode of stroke were lost in the PPARα gene-disrupted transgenic mouse (Figure 3).
The synthetic high-affinity cannabinoid agonist WIN 55212-2 displayed higher PPARα-binding affinity than OEA, although with biphasic effects on PPARα gene-transcription activity. WIN 55212-2 has also been found to display biphasic effects on CB receptors. For example, CB1-evoked voltage-dependent currents in retinal cones were enhanced by low concentrations (<1 μM) and inhibited by high concentrations (>1 μM) of WIN 55212-2 (Fan and Yazulla, 2003). This novel discovery that WIN 55212-2 has the ability to bind to and activate PPARα might provide a new way to explain, at least in part, its complex biological profile.
Although widely accepted as an endocannabinoid, anandamide has also been found to activate TRPV1 receptors (Zygmunt et al., 1999) and PPARγ (Bouaboula et al., 2005; O'Sullivan et al., 2005). By using the ligand binding and transactivation assays (Figures 1 and 2), anandamide was further identified as a weak PPARα ligand. These multiple receptor targets of anandamide might confound its effects in various biological systems. For example, anandamide might induce apoptosis in the HeLa cells via TRPV1 (Contassot et al., 2004), which could obscure its effects on PPARα-transcription activity. Since these receptors and related pathways have different distributions in the body, anandamide effects in vivo could be expected to display tissue specificity. This is consistent with the manifold effects evoked by anandamide in the CNS and periphery. For example, it controls pain initiation, secretion of pituitary hormones, wake/sleep cycles, thermogenesis and appetite in the brain; in the cardiovascular system, anandamide profoundly decreases blood pressure and heart rate, reduces sympathetic tone due to inhibition of noradrenaline release and induces vasodilatation; in the immune system, anandamide supresses interleukin-2 transcription and secretion, stimulates interleukin-6 synthesis and inhibits tumour necrosis factor-α production (Maccarrone and Finazzi-Agro, 2002, 2003).
The putative endocannabinoids, noladin ether and virodhamine, were found to show similar binding and transcriptional activity at PPARα as anandamide. Although these three agents are all arachidonic acid derivatives, they exhibit dissimilar affinities and efficacies for CB1 and TRPV1 receptors (Duncan et al., 2004; Steffens et al., 2005). In addition, we observed that noladin ether and virodhamine were less toxic to HeLa cells than anandamide (data not shown).
Tetrahydrocannabinol, the main psychoactive component in the cannabis plant, on the other hand, was found to lack significant PPARα binding or transcription activation. THC has been shown to act on PPARγ and stimulate adipocyte differentiation in cultured 3T3L1 cells, in common with other PPARγ ligands (Bouaboula et al., 2005; O'Sullivan et al., 2005).
In vivo effects of cannabinoids: mechanisms of neuroprotection
On the basis of in vitro assays of receptor occupancy and gene transcription, we identified that the synthetic cannabinoid WIN 55212-2 and the endogenous cannabinoid-like compound OEA were both capable of PPARα activation (Figures 1 and 2). We chose to investigate next whether these agents had PPARα agonist activity in vivo. Thus, although virodhamine, noladin and anandamide exhibited similar potency to OEA in competing for CPA binding to the PPARα LBD (Figure 1b), and evoked similar gene-transcription responses in the reporter gene assay (Figure 2), OEA was chosen for further investigation to avoid the potential for CB1 or CB2 cannabinoid receptor activation (Lin et al., 1998) and subsequent neuroprotective effects (Nagayama et al., 1999). In pilot experiments for another study, we have observed that OEA administration leads to a marked increase in tissue levels of OEA, without alterations in anandamide levels (manuscript in preparation). Thus, while it is possible that fatty acid amide hydrolase-mediated hydrolysis of OEA, and the subsequent production of oleic acid, contributes to the activation of PPARα in intact systems, the use of fatty acid amide hydrolase-null is complicated by the elevations of multiple ECLs, including 10-fold increased levels of anandamide (an agonist at CB1, CB2, PPARα and PPARγ receptors) and 20-fold increased levels of OEA (an agonist at PPARα receptors) (Clement et al., 2003).
Furthermore, from the amounts of OEA administered, calculated to be 0.9 nmol per mouse based on a 30 g mouse, this could conceivably provide a plasma concentration of 0.5 μM, which is considerably less than circulating plasma FFA levels (∼400 μM), approximately one-quarter of which is oleate (Seo et al., 2002). It seems unlikely, therefore, that any action of exogenous OEA is mediated exclusively through fatty acid amide hydrolase-mediated hydrolysis to oleate.
In in vivo models, OEA and WIN 55212-2 were found to increase lipolysis significantly in mice, while noladin ether showed a strong trend towards enhancement (Figure 4a). The lipolytic effect of OEA has previously been confirmed to function through PPARα (Guzman et al., 2004). The lipolytic effect of WIN 55212-2, which was greater than the effects caused by other cannabinoids, might also be due to PPARα activation, despite being apparently less effective in the in vitro model (Figure 2). A preliminary study with PPARα-null mice failed to show a significant elevation of plasma FFA following WIN 55212-2 administration (data not shown).
In the mouse stroke model, both fenofibrate (Deplanque et al., 2003) and OEA (Figure 3) were found to have neuroprotective activities through PPARα, since they were unable to alter infarct volume in PPARα-deficient mice. Since the neuroprotective effects of PPARα were suggested to be independent of its well-known lipid-lowering effects (Deplanque et al., 2003), antioxidant and anti-inflammatory mechanisms might underlie PPARα-dependent neuroprotection. A likely route for PPARα regulation of inflammatory responses is via repression of NFκB signalling (Staels et al., 1998). The NF-κB pathway plays an important role in the immune system and is generally thought to exacerbate brain damage in ischaemic stroke. In mouse cerebral cortex, the NF-κB inhibitory protein IκBα was induced by OEA treatment (Figure 4b) and thus would be expected to restrict activity in the NF-κB pathway, inhibition of which has been demonstrated to reduce brain damage in several ischaemic stroke models (Salminen et al., 1995; Yang et al., 1995; Schneider et al., 1999). Consistent with these findings, expression of the NF-κB-regulated COX-2 gene was found to be reduced by OEA treatment. Inhibition of COX-2 might contribute to OEA neuroprotection against stroke, since this enzyme is responsible for the production of prostaglandins that can potentiate pain and inflammation. The expression of COX-2 is generally found to be upregulated after stroke (Miettinen et al., 1997; Nogawa et al., 1997), indicating a potential route for therapeutic intervention following such an insult. Although its role in cerebral ischaemic damage in man is unclear, inhibition of COX-2 expression can reduce the infarct volume and neuronal damage in stroke models (Iadecola et al., 2001). For example, selective inhibition of COX-2 has been shown to reduce markedly global ischaemia-evoked hippocampal neuronal death (Nakayama et al., 1998). However, given the network of genes that the NF-κB pathway is known to influence in the CNS, including neural cell adhesion molecules, amyloid precursor protein, μ-opioid receptors, brain-derived neurotrophic factor, manganese-dependent superoxide dismutase and Ca2+/calmodulin-dependent protein kinase II (O'Neill and Kaltschmidt, 1997), it is unlikely that COX-2 is solely responsible for PPARα-regulated neuroprotection.
There is evidence that CB1 receptor-mediated neuroprotection might also be mediated, at least in part, through the NF–κB pathway (Panikashvili et al., 2005), and so the possibility exists of generating/developing compounds with dual CB1/PPARα activity which converge at the level of cellular regulation through this important transcription factor.
Implications of PPARα as a target for cannabinoids
Our results, which show that multiple cannabinoid receptor ligands are also agonists at PPARα nuclear receptors, raise the issue that PPARα seems less fastidious with regard to ligand structures than cell-surface 7-transmembrane receptors (such as CB1 or CB2) or transmitter-gated channels like TRPV1. The large ligand-binding pocket of PPARα, which is able to accommodate a wide variety of fatty acid-derived molecules, is presumably the reason underlying such ligand promiscuity (Wang et al., 2004).
Intriguingly, there appear to be differential effects of cannabinoid ligands on PPARs, in which THC appears to be a PPARγ-selective ligand (Figures 1 and 2; O'Sullivan et al., 2005), while WIN 55212-2 is able to activate both PPARα and PPARγ (Figures 1 and 2, and data not shown). It is tempting to speculate, therefore, that this variation may explain, at least in part, the variation in cannabinoid action in vivo.
Cannabinoid ligands are generally thought to have the ability to control appetite (Cota et al., 2003a), while many hypolipidaemic drugs are identified as PPARα ligands. On the other hand, some cannabinoids, such as THC and anandamide, were found to stimulate adipocyte differentiation through PPARγ (Bouaboula et al., 2005; O'Sullivan et al., 2005). Since PPARα and PPARγ play divergent roles in lipid homeostasis, agonists with dual or triple PPAR and CB receptor activities may have potential in dyslipidaemia therapy by targeting both CNS and periphery pathways. Alternatively, the discovery of PPAR-selective cannabinoids may lead to the development of new antidiabetic and hypolipidaemic drugs. Since OEA was only neuroprotective following repeated treatment in advance of ischaemic challenge, it could be argued that OEA, or agents working through the same mechanism, would not be practically useful as stroke medicines. However, there are populations of patients at increased risk of stroke (for example, following repeated incidence of transient ischaemic attacks) who could benefit from prophylactic treatment. The ability of OEA, given as a repeated treatment after ischaemia, to attenuate or reverse brain damage was not determined in the present study.
In summary, the data presented here provide strong evidence that selected cannabinoids (WIN 55212-2, OEA, noladin ether and virodhamine) are PPARα agonists, and suggest a novel means by which the multiple effects of cannabinoids, in both the CNS and periphery, could be brought about. In addition to its well-recognized role in lipid metabolism, PPARα activation showed obvious beneficial effects in ischaemic brain damage, which is likely to be connected with its anti-inflammatory action through the NF–κB pathway. These discoveries not only broaden the potential use of cannabinoids as therapeutic agents, but also support PPARα as a new target for neuroprotective treatment.
Acknowledgments
We thank Dr Kostas Tzintzas (Biomedical Sciences, University of Nottingham) for assistance with the measurement of fatty acids and Professor J Stephen Clark (Chemistry, University of Nottingham, now University of Glasgow) for provision of synthetic chemistry facilities.
Abbreviations
- CPA
cis-parinaric acid
- ECL
endogenous cannabinoid-like molecules
- LBD
ligand-binding domain
- OEA
N-oleoylethanolamine
- PPAR
peroxisome proliferator-activated receptor
- Δ9THC
tetrahydrocannabinol
Conflict of interest
The authors state no conflict of interest.
References
- Baker D, Pryce G, Davies WL, Hiley CR. Insilico patent searching reveals a new cannabinoid receptor. Trends Pharmacol Sci. 2006;27:1–4. doi: 10.1016/j.tips.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Berger JP, Akiyama TE, Meinke PT. PPARs: therapeutic targets for metabolic disease. Trends Pharmacol Sci. 2005;26:244–251. doi: 10.1016/j.tips.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Hilairet S, Marchand J, Fajas L, Fur GL, Casellas P. Anandamide induced PPARγ transcriptional activation and 3T3-L1 preadipocyte differentiation. Eur J Pharmacol. 2005;517:174–181. doi: 10.1016/j.ejphar.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breivogel CS, Griffin G, Di Marzo V, Martin BR. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol. 2001;60:155–163. [PubMed] [Google Scholar]
- Burstein S. PPAR-γ: a nuclear receptor with affinity for cannabinoids. Life Sci. 2005;77:1674–1684. doi: 10.1016/j.lfs.2005.05.039. [DOI] [PubMed] [Google Scholar]
- Causevic M, Wolf CR, Palmer CNA. Substitution of a conserved amino acid residue alters the ligand binding properties of peroxisome proliferator activated receptors. FEBS Lett. 1999;463:205–210. doi: 10.1016/s0014-5793(99)01618-x. [DOI] [PubMed] [Google Scholar]
- Clement AB, Hawkins EG, Lichtman AH, Cravatt BF. Increased seizure susceptibility and proconvulsant activity of anandamide in mice lacking fatty acid amide hydrolase. J Neurosci. 2003;23:3916–3923. doi: 10.1523/JNEUROSCI.23-09-03916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contassot E, Tenan M, Schnuriger V, Pelte MF, Dietrich PY. Arachidonyl ethanolamide induces apoptosis of uterine cervix cancer cells via aberrantly expressed vanilloid receptor-1. Gynecol Oncol. 2004;93:182–188. doi: 10.1016/j.ygyno.2003.12.040. [DOI] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Lutz B, Vicennati V, Stalla GK, Pasquali R, et al. Endogenous cannabinoid system as a modulator of food intake. Int J Obes Relat Metab Disord. 2003a;27:289–301. doi: 10.1038/sj.ijo.0802250. [DOI] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003b;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deplanque D, Gele P, Petrault O, Six I, Furman C, Bouly M, et al. Peroxisome proliferator-activated receptor-α activation as a mechanism of preventive neuroprotection induced by chronic fenofibrate treatment. J Neurosci. 2003;23:6264–6271. doi: 10.1523/JNEUROSCI.23-15-06264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov. 2004;3:771–784. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L, Fezza F, Ligresti A, Bisogno T. Anandamide receptors. Prostaglandins Leukot Essent Fatty Acids. 2002;66:377–391. doi: 10.1054/plef.2001.0349. [DOI] [PubMed] [Google Scholar]
- Duncan M, Millns P, Smart D, Wright JE, Kendall DA, Ralevic V. Noladin ether, a putative endocannabinoid, attenuates sensory neurotransmission in the rat isolated mesenteric arterial bed via a non-CB1/CB2 Gi/o linked receptor. Br J Pharmacol. 2004;142:509–518. doi: 10.1038/sj.bjp.0705789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan SF, Yazulla S. Biphasic modulation of voltage-dependent currents of retinal cones by cannabinoid CB1 receptor agonist WIN 55212-2. Vis Neurosci. 2003;20:177–188. doi: 10.1017/s095252380320208x. [DOI] [PubMed] [Google Scholar]
- Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez De Fonseca F, et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-α. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- Gibson CL, Murphy SP. Progesterone enhances functional recovery after middle cerebral artery occlusion in male mice. J Cereb Blood Flow Metab. 2004;24:805–813. doi: 10.1097/01.WCB.0000125365.83980.00. [DOI] [PubMed] [Google Scholar]
- Guzman M, Lo Verme J, Fu J, Oveisi F, Blazquez C, Piomelli D. Oleoylethanolamide stimulates lipolysis by activating the nuclear receptor peroxisome proliferator-activated receptor α (PPAR-α) J Biol Chem. 2004;279:27849–27854. doi: 10.1074/jbc.M404087200. [DOI] [PubMed] [Google Scholar]
- Hajos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106:1–4. doi: 10.1016/s0306-4522(01)00287-1. [DOI] [PubMed] [Google Scholar]
- Howlett AC. Pharmacology of cannabinoid receptors. Annu Rev Pharmacol Toxicol. 1995;35:607–634. doi: 10.1146/annurev.pa.35.040195.003135. [DOI] [PubMed] [Google Scholar]
- Howlett AC. The cannabinoid receptors. Prostaglandins Other Lipid Mediat. 2002;68–69:619–631. doi: 10.1016/s0090-6980(02)00060-6. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Niwa K, Nogawa S, Zhao X, Nagayama M, Araki E, et al. Reduced susceptibility to ischemic brain injury and N-methyl-D-aspartate-mediated neurotoxicity in cyclooxygenase-2-deficient mice. Proc Natl Acad Sci USA. 2001;98:1294–1299. doi: 10.1073/pnas.98.3.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, et al. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci USA. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson KO, Vandevoorde S, Lambert DM, Tiger G, Fowler CJ. Effects of homologues and analogues of palmitoylethanolamide upon the inactivation of the endocannabinoid anandamide. Br J Pharmacol. 2001;133:1263–1275. doi: 10.1038/sj.bjp.0704199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert DM, DiPaolo FG, Sonveaux P, Kanyonyo M, Govaerts SJ, Hermans E, et al. Analogues and homologues of N-palmitoylethanolamide, a putative endogenous CB2 cannabinoid, as potential ligands for the cannabinoid receptors. Biochim Biophys Acta. 1999;1440:266–274. doi: 10.1016/s1388-1981(99)00132-8. [DOI] [PubMed] [Google Scholar]
- Lin SY, Khanolkar AD, Fan PS, Goutopoulos A, Qin C, Papahadjis D, et al. Novel analogues of arachidonylethanolamide (anandamide): affinities for the CB1 and CB2 cannabinoid receptors and metabolic stability. J Med Chem. 1998;41:5353–5361. doi: 10.1021/jm970257g. [DOI] [PubMed] [Google Scholar]
- Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, et al. The nuclear receptor peroxisome proliferator-activated receptor-α mediates the antiinflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005;67:15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Finazzi-Agro A. Endocannabinoids and their actions. Vitam Horm. 2002;65:225–255. doi: 10.1016/s0083-6729(02)65066-6. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Finazzi-Agro A. The endocannabinoid system, anandamide and the regulation of mammalian cell apoptosis. Cell Death Differ. 2003;10:946–955. doi: 10.1038/sj.cdd.4401284. [DOI] [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptors as therapeutic targets. Annu Rev Pharmacol Toxicol. 2006;46:101–122. doi: 10.1146/annurev.pharmtox.46.120604.141254. [DOI] [PubMed] [Google Scholar]
- Miettinen S, Fusco FR, Yrjanheikki J, Keinanen R, Hirvonen T, Roivainen R, et al. Spreading depression and focal brain ischemia induce cyclooxygenase-2 in cortical neurons through N-methyl-D-aspartic acid-receptors and phospholipase A2. Proc Natl Acad Sci USA. 1997;94:6500–6505. doi: 10.1073/pnas.94.12.6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama T, Sinor AD, Simon RP, Chen J, Graham SH, Jin KL, et al. Cannabinoids and neuroprotection in global and focal cerebral ischemia and in neuronal cultures. J Neurosci. 1999;19:2987–2995. doi: 10.1523/JNEUROSCI.19-08-02987.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M, Uchimura K, Zhu RL, Nagayama T, Rose ME, Stetler RA, et al. Cyclooxygenase-2 inhibition prevents delayed death of CA1 hippocampal neurons following global ischemia. Proc Natl Acad Sci USA. 1998;95:10954–10959. doi: 10.1073/pnas.95.18.10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogawa S, Zhang F, Ross ME, Iadecola C. Cyclo-oxygenase-2 gene expression in neurons contributes to ischemic brain damage. J Neurosci. 1997;17:2746–2755. doi: 10.1523/JNEUROSCI.17-08-02746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill LA, Kaltschmidt C. NF-κB: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997;20:252–258. doi: 10.1016/s0166-2236(96)01035-1. [DOI] [PubMed] [Google Scholar]
- O'Sullivan SE, Tarling EJ, Bennett AJ, Kendall DA, Randall MD. Novel time-dependent vascular actions of Δ9-tetrahydrocannabinol mediated by peroxisome proliferator-activated receptor γ. Biochem Biophys Res Commun. 2005;337:824–831. doi: 10.1016/j.bbrc.2005.09.121. [DOI] [PubMed] [Google Scholar]
- Overton HA, Babbs AJ, Doel SM, Fyfe MC, Gardner LS, Griffin G, et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006;3:167–175. doi: 10.1016/j.cmet.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Panikashvili D, Mechoulam R, Beni SM, Alexandrovich A, Shohami E. CB1 cannabinoid receptors are involved in neuroprotection via NF-κB inhibition. J Cereb Blood Flow Metab. 2005;25:477–484. doi: 10.1038/sj.jcbfm.9600047. [DOI] [PubMed] [Google Scholar]
- Salminen A, Liu PK, Hsu CY. Alteration of transcription factor binding activities in the ischemic rat brain. Biochem Biophys Res Commun. 1995;212:939–944. doi: 10.1006/bbrc.1995.2060. [DOI] [PubMed] [Google Scholar]
- Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M. NF-κB is activated and promotes cell death in focal cerebral ischemia. Nat Med. 1999;5:554–559. doi: 10.1038/8432. [DOI] [PubMed] [Google Scholar]
- Seo T, Velez-Carrasco W, Qi K, Hall M, Worgall TS, Johnson RA, et al. Selective uptake from LDL is stimulated by unsaturated fatty acids and modulated by cholesterol content in the plasma membrane: role of plasma membrane composition in regulating non-SR-BI-mediated selective lipid transfer. Biochemistry. 2002;41:7885–7894. doi: 10.1021/bi011949g. [DOI] [PubMed] [Google Scholar]
- Staels B, Koenig W, Habib A, Merval R, Lebret M, Torra IP, et al. Activation of human aortic smooth-muscle cells is inhibited by PPARα but not by PPARγ activators. Nature. 1998;393:790–793. doi: 10.1038/31701. [DOI] [PubMed] [Google Scholar]
- Steffens M, Zentner J, Honegger J, Feuerstein TJ. Binding affinity and agonist activity of putative endogenous cannabinoids at the human neocortical CB1 receptor. Biochem Pharmacol. 2005;69:169–178. doi: 10.1016/j.bcp.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Turk M, Dincer S, Yulug IG, Piskin E. In vitro transfection of HeLa cells with temperature sensitive polycationic copolymers. J Control Release. 2004;96:325–340. doi: 10.1016/j.jconrel.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Wang MM, Winneroski LL, Ardecky RJ, Babine RE, Brooks DA, Etgen GJ, et al. Conversion of human-selective PPAR alpha agonists to human/mouse dual agonists: a molecular modeling analysis. Bioorg Med Chem Lett. 2004;14:6113–6116. doi: 10.1016/j.bmcl.2004.09.031. [DOI] [PubMed] [Google Scholar]
- Yang K, Mu XS, Hayes RL. Increased cortical nuclear factor-kappa B (NF-κB) DNA binding activity after traumatic brain injury in rats. Neurosci Lett. 1995;197:101–104. doi: 10.1016/0304-3940(95)11919-n. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang HH, Sørgård M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]