Fig. 7.
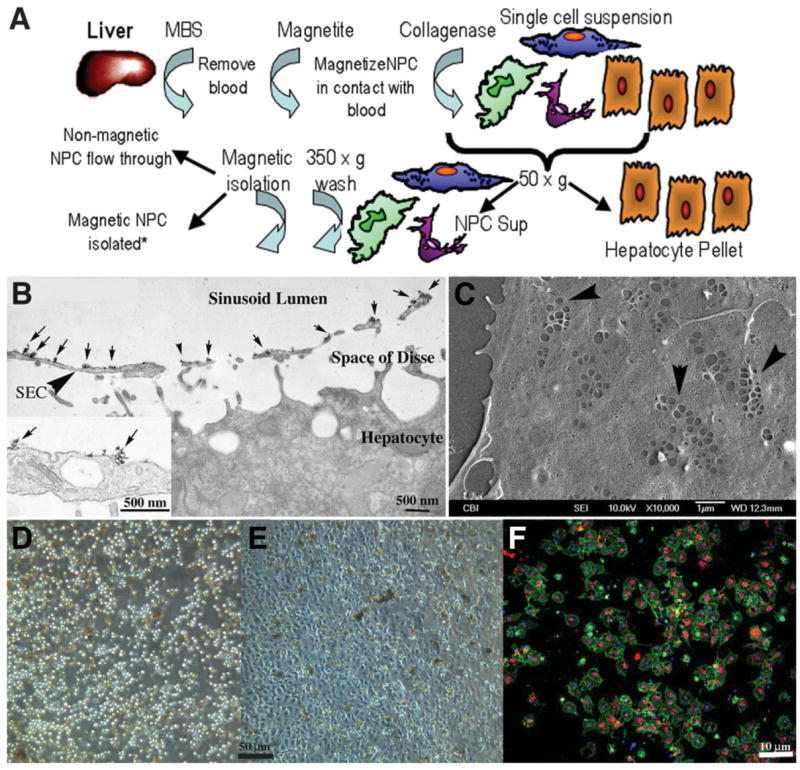
(A) Schematic flow diagram outlining isolation of liver sinusoidal NPCs following absorption of cationic magnetite to sinusoidal NPC surfaces. Magnetite perfused through the cleared liver remains adsorbed to NPC membrane surfaces following collagenase perfusion, allowing for specific isolation by MACs columns. *Other liver cells such as hepatocytes, Kupffer cells, and stellate cells can also be isolated and cultured via negative or positive selection (Kupffer cell panning) from the original cell suspension. (B) Transmission electron micrograph of liver fixed immediately after perfusion with cationic colloidal magnetite. Arrows indicate particles of cationic magnetite adsorbed to apical sinusoidal endothelial cell membrane surfaces (arrowhead, SEC). Magnetite particles do not cross through the fenestrated endothelium and do not adsorb to proteins within the space of Disse or the basolateral membrane of the hepatocyte. Inset shows high magnification of magnetite particle clusters on the luminal endothelial cell surface. (C) Scanning electron micrograph of LSECs 24 hours after plating onto collagen-adsorbed coverslip. SECs retain their fenestrations in characteristic sieve plate arrangements (arrowheads). (D) Phase micrograph of original LSEC isolate obtained from the MACs column and plated onto collagen-adsorbed coverslips showing homogeneous cell size. (E) The same isolate 24 hours after plating showing the characteristic cobblestone appearance of endothelium. (F) Uptake of Di-I-acetylated LDL (red) and coincident staining of rat LSEC-specific marker SE-1 (green) in magnetite-isolated LSECs in 24-hour cultures (blue staining indicates Hoechst’s dye-stained nuclei).
