Figure 3.
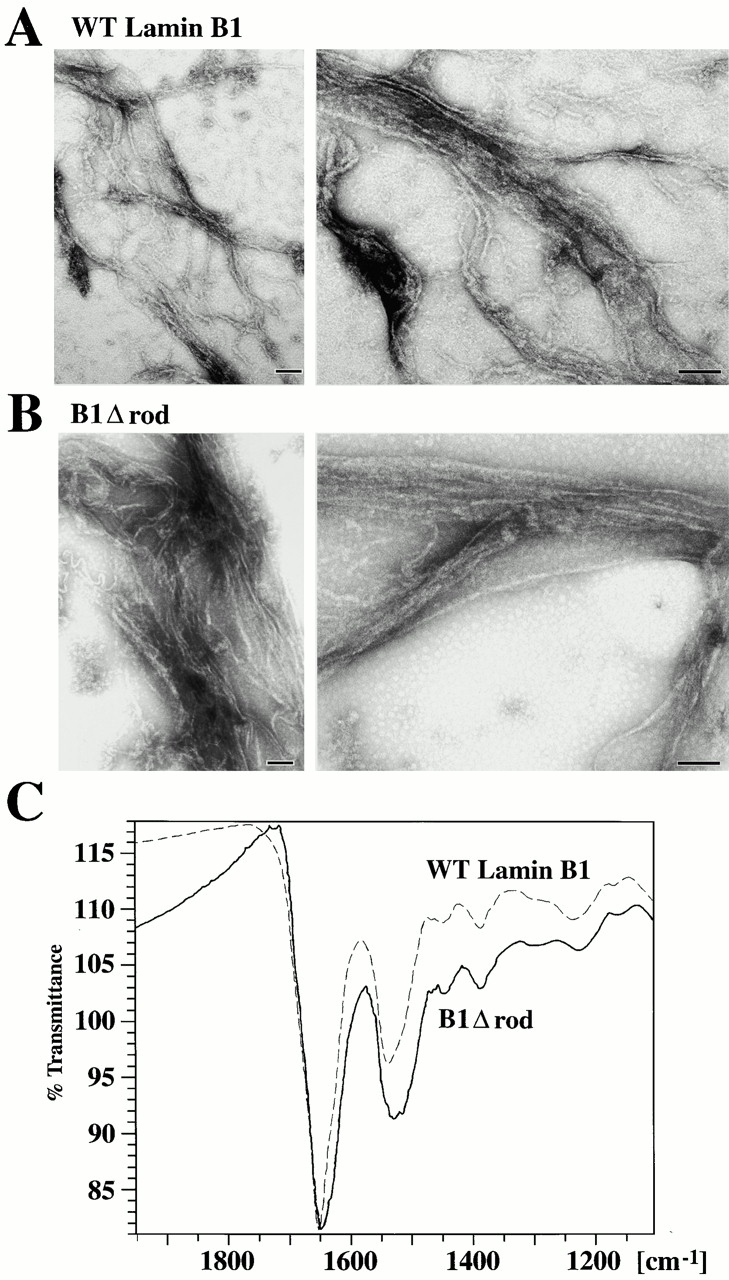
In vitro assembly of B1Δrod. (A–B) WT lamin B1 and B1Δrod that were dialyzed out of urea-formed filaments. The structures formed by each protein were similar in appearance (bars, 100 nm). (C) FTIR indicates α-helical structures occur in the filamentous form of B1Δrod, similar to WT lamin B1. The proteins were dialyzed using conditions that had yielded filaments by EM analysis. Polymerized material was pelleted and analyzed by FTIR. The scans of B1Δrod and WT lamin B1 were very similar although the band around 1650 was wider for B1Δrod than for WT lamin B1. The position of the bands is consistent with α-helical rather than β-sheet characteristics (see text).
