Figure 7.
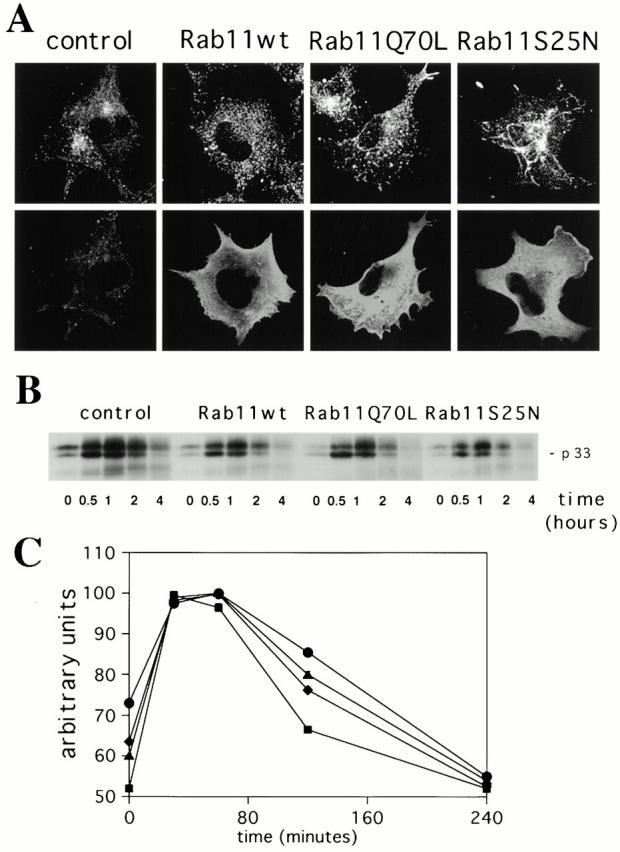
Overexpression of Rab11wt and mutant proteins does not alter the transport of the αβIi precursor forms of MHC class II molecules from the TGN to degradative compartments in the M10 melanoma cell line. (A) M10 cells were transfected for 5 h with empty pGEM plasmid (control cells), Rab11wt, Rab11Q70L, or Rab11S25N plasmids. Cells were double labeled for TfR (top) and Rab11 (bottom) as described in the legend to Fig. 1. (B) Cells were pulse labeled for 10 min. After the indicated times of chase, the L243 mAb was used for the first immunoprecipitation in order to remove newly generated αβ dimers. Proteins in the supernatants of the first immunoprecipitation were then immunoprecipitated with the DA6.147 mAb. DA6.147 immunoprecipitates, which contain αβ dimers associated with Ii, were treated for SDS-PAGE. Coprecipitated Ii (p33) is indicated. No differences were detected among the cell types tested. The figure is representative of three different experiments. Note that oligomerization of the αβIi complexes was complete only 30 min after the pulse and that degradation of p33-associated Ii did not vary significantly within the different overexpressing cells. (C) The absence of an effect from Rab11wt and mutants overexpression in M10 cells on the degradation of Ii was confirmed by the quantification of the signal detected for the p33 Ii in each lane (control, •; Rab11Q70L, ♦; Rab11wt, ▴; Rab11S25N, ▪). Results are representative of three distinct experiments. Data are expressed as a percentage of the maximum Ii associated with αβ dimers found at the 30-min time point.
