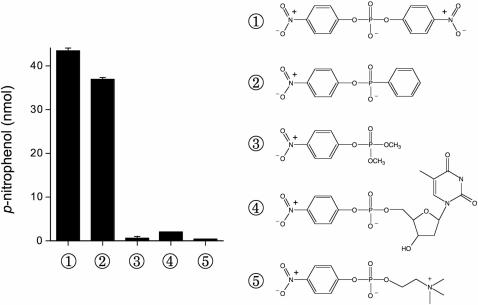
Figure 2.
Substrate specificity of CthPnkp. Reaction mixtures (25 µl) containing 50 mM Tris–HCl (pH 7.5), 0.5 mM MnCl2, 1.5 µg of CthPnkp and 10 mM substrate as specified were incubated for 30 min at 45°C. The reactions were quenched by adding 20 mM EDTA and then 0.9 ml of 1 M Na2CO3. Release of p-nitrophenol was determined by measuring A410 and interpolating the value to a p-nitrophenol standard curve. The extents of formation of p-nitrophenol are plotted at left. The chemical structures of the substrates bis-p-nitrophenyl phosphate ①, p-nitrophenyl phenylphosphonate ②, dimethyl-p-nitrophenyl phosphate ③, thymidine 5′-monophosphate-p-nitrophenyl ester ④ and p-nitrophenyl phosphorylcholine ⑤ are shown at right.