Figure 8.
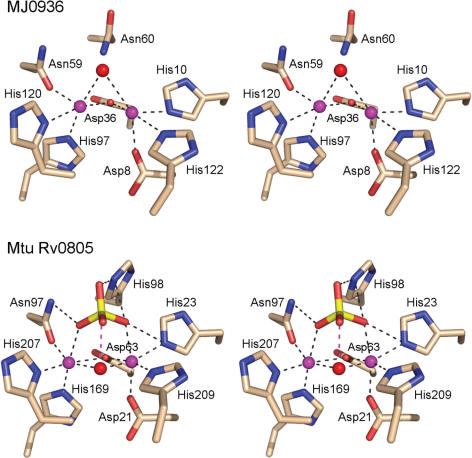
Active sites of binuclear metallophosphodiesterases MJ0936 and Rv0805. Stereo views of the active site of Methanoccus jannaschii MJ0936 (top panel; PDB 1S3N) and Mycobacterium tuberculosis Rv0805 (bottom panel; PDB 2HY1) oriented similarly to the λ-Pase active site depicted in Figure 1. The amino acid side chains coordinating the binuclear metal cluster (and the phosphate ion in Rv0805) are shown. The metal ions are colored magenta. Water is colored red. Unlike CthPnkp and λ-Pase, the MJ0936 and Rv0805 phosphodiesterases have no arginine side chain in their active sites. Whereas the cyclic nucleotide phosphodiesterase Rv0805 has a phosphate-coordinating histidine corresponding to CthPnkp His264, the analogous residue is asparagine in MJ0936, which is unable to hydrolyze cyclic nucleotides. The water-mediated metal contact of Asp202 in λ-Pase is replaced by a direct interaction of the metal with a histidine in both MJ0936 and Rv0805.