Abstract
Context: Over the years, different hypotheses involving the ovarian steroid hormones have been proposed to explain the luteal phase occurrence of severe premenstrual syndrome symptoms. Although it had been strongly suspected that differences in the concentrations of the ovarian steroids may underlie the mood and psychological imbalance of this disorder, the evidence for this hypothesis has been inconsistent and remains controversial.
Objective: Our objective was to measure the ovarian steroid hormones across the menstrual cycle in women with and without luteal phase symptoms consistent with premenstrual dysphoric disorder (PMDD).
Design: We measured estradiol (E2), progesterone, and SHBG in women with and without PMDD using a cross-sectional and prospective experimental design. Participating women underwent 2-month self-assessment symptom screening and 1-month hormonal evaluation.
Results: Overall means for LH, progesterone, E2, peak E2, and free E2 were not different between groups. Across the menstrual cycle, overall percent free E2 was significantly lower and SHBG significantly greater in the PMDD group compared with controls (1.39 ± 0.26 vs. 1.50 ± 0.28, P = 0.03; 61.4 ± 25.1 vs. 52.4 ± 21.3 nmol/liter, P = 0.046, respectively). During the luteal phase, free E2 was significantly lower in the PMDD group compared with controls (PMDD 7.6 ± 7.0 vs. controls 8.9 ± 8.4 pmol/liter; P = 0.032). For both follicular and luteal phases, SHBG was significantly higher in the PMDD group (follicular phase 60.5 ± 31.7 vs. 51.4 ± 38.2 nmol/liter, P = 0.047; luteal phase 65.1 ± 32.3 vs. 55.1 ± 38.9 nmol/liter, P =0.03). In both groups, SHBG significantly increased from the follicular to luteal phase.
Conclusion: Luteal phase concentrations of free E2, percent free E2, and SHBG differ significantly between women with and without PMDD.
Premenstrual Dysphoric Disorder (PMDD) is a poorly understood feminine disorder. A study measuring the ovarian steroid hormones across the menstrual cycle in women with and without this disorder reveals lower free estradiol (E2), lower %free E2, and higher SHBG during the luteal phases in PMDD women.
Premenstrual dysphoric disorder (PMDD), also described as severe premenstrual syndrome (PMS), is a poorly understood disorder characterized by the cyclical occurrence of behavioral, psychiatric, and physical symptoms during the luteal phase of the menstrual cycle. Women with PMDD experience mood changes, irritability, and depression so severe that personal and professional role performance is frequently disrupted.
Severe PMS is unique to premenopausal women because it is related to the ovarian cycle and does not occur before puberty, during pregnancy, or after menopause. Indeed, there appears to be compelling evidence that PMDD is related to the ovarian hormonal rhythmicities of the menstrual cycle (1,2,3), as symptom relief ensues with suppression of ovulation and ablation of these cyclical ovarian hormonal changes. Bilateral ovariectomy, estradiol (E2) implants, oral contraceptives, and GnRH agonists have significantly diminished PMS symptoms (4,5,6,7,8). However, the precise role of the ovarian steroid hormones remains a controversial one because most investigations have failed to discern any overall difference in ovarian hormone concentrations across the menstrual cycle. However, several studies have reported higher luteal phase E2 concentrations, whereas others have reported, in contrast, lower concentrations between women with and without PMDD (9,10,11,12,13).
In view of the fact that estrogen has a profound effect on mood (14), we expected that women with PMDD would have lower luteal phase free E2 concentrations compared with healthy asymptomatic controls. Our aims were to: 1) measure fluctuations in total, free, and bound ovarian steroid hormone levels across the menstrual cycle in women with and without PMDD; and 2) determine whether differences in the ovarian steroid hormones existed between these two groups of women.
Subjects and Methods
Study subjects
Recruitment and study design have previously been described (15). This investigation, conducted between 2000 and 2005 was approved by the institutional review board of St. Luke’s-Roosevelt Hospital Center. All participants provided written informed consent. Briefly, healthy, premenopausal women between the ages of 18 and 45 yr, with either severe PMS or asymptomatic menstrual cycles were recruited from the New York City metropolitan and tristate area. Inclusion criteria for all participants were general good health and regular menstrual cycles 21–35 d in length (+5 d). Exclusion criteria for women recruited to either group were: current major medical condition or serious medical illness in the past; major psychiatric disorder, including active depression; menstrual cycle irregularity; oral contraceptive medication; bone and calcium disorders; and any overt metabolic disorder, such as diabetes mellitus. Prescreening by telephone excluded subjects not meeting inclusion and exclusion criteria. To be included as an asymptomatic control required a woman to have: 1) no prior medical history of PMDD or PMS, 2) a prospective and consecutive assessment with a 2-month daily diary demonstrating minimal symptoms during both the luteal and intermenstrual phases with no evidence of functional impairment, and 3) no more than two symptoms rated in either the moderate or severe range on 3 or more days during any one screening cycle. For the PMDD group, a woman required a diagnosis of PMDD meeting the Diagnostic and Statistical Manual of Mental Disorders: DSM-IV (16) criteria based upon a prospective and consecutive self-assessment with a 2-month daily symptom diary. Inclusion diagnosis for the PMDD group required five or more symptoms rated at least moderate in severity for the last week of the menstrual cycle with evidence of functional impairment for at least 2 d and a minimal symptom intermenstrual phase occurring after menses.
Protocol design
Eligibility evaluation included a medical, menstrual, and gynecological history, as well as a physical examination and baseline laboratory screening with complete blood count, chemistry panel, thyroid function panel, pregnancy test, and 24-h urine calcium measurement. Two months of prospective, consecutive daily symptom screening with 1-month hormonal evaluation were conducted. If two consecutive menstrual cycles did not fulfill the inclusion criteria, the asymptomatic control candidate was disqualified. PMDD participants were allowed three menstrual cycles to qualify, if either of the first 2 screening months did not meet inclusion criterion. Daily symptom assessment continued throughout the menstrual cycle when hormonal evaluation occurred. A modified structured psychiatric interview (Structured Clinical Interview for DSM-III-R) was conducted to exclude current or recent mental disorders within the previous 6 months (17).
Symptom assessment
The PMS diary, a self-assessment symptom rating scale, was used for the symptom screening, monitoring, and diagnosis of both the PMDD and control participants (18). The PMS diary is a concise, validated questionnaire comprised of 18 physical and emotional symptoms: 11 items with item 11 having eight physical symptoms. Each symptom is rated on a four-point rating scale: 0, absent; 1, mild; 2, moderate; and 3, severe. The average of the components of symptom 11 counted as a single item when determining qualification for inclusion criteria, whereas the severity rating of all 18 symptom items were summed for a total symptom severity score. The luteal mean was defined as the 7-d symptom mean before the onset of menstruation. The menstrual mean was defined as the mean scores during days of menstruation and the intermenstrual mean as the 7-d symptom mean immediately after menses.
Measurements
Timed hormone and biochemical samples across the menstrual cycle were drawn based on the length of the qualifying subject’s two previous menstrual cycles. Serum and urine samples were collected at eight points in time (i.e. d 2, 7, 12–15, 22, and 27 based on a 28- to 30-d cycle). Two samples were obtained within the early follicular phase, four within the periovulatory period, and two samples within the mid-to-late luteal phase. The timing and frequency of the blood samples were adjusted according to the average duration of the individual subject’s two qualifying menstrual cycles. Serum samples were assayed for E2 with the extraction chromatography RIA (normal range depending on phase, 20–750 pg/ml), progesterone with chemiluminescence assay (normal range depending on phase, 50–2500 ng/dl), and LH with immunochemiluminometric assay (normal range 0.5–76.3 IU/liter). SHBG was measured with the immunochemiluminescent assay (normal range 17–120 nmol/liter; intraassay and interassay variations 4.3 and 4.7%, respectively). Laboratory tests were performed at Nichols Institute, Quest Diagnostics (San Juan Capistrano, CA). Blood samples were collected between 0700 and 1000 h after an overnight fast. Blood samples were allowed to clot for 1 h at room temperature, and serum was obtained by centrifugation. All timed hormone samples were frozen at −70 C and assayed simultaneously for an individual subject. Ovulation was determined by the LH surge and the highest E2 level, on examination and review of E2 and LH levels from d 10–14 along with a minimum luteal phase serum progesterone concentration of 500 ng/dl.
Statistical analysis
Descriptive statistics were calculated with means, sd values, and 95% confidence intervals for all continuous measures and counts and percentages for categorical measures. Dependent measures were grouped into content domains (demographics, reproductive histories, habitus, serum chemistries, and urine chemistries). LH and progesterone were log transformed before analysis and reported in untransformed units. To maintain a family-wise fixed type I error rate, a multivariate ANOVA model for the measures within each content domain was used to test the hypothesis that PMDD and control groups differed on at least one measure within a content domain, and a P value less than 0.05 for the fixed effect of group was used to warrant univariate analysis of group differences. Univariate tests of baseline differences between PMDD and control groups used Student’s t tests for continuous measures or Fisher’s exact test for categorical variables. Differences between PMDD and controls in the temporal pattern of repeated measures were estimated with linear mixed models (SAS Proc MIXED; SAS Institute Inc., Cary, NC). Here, group (PMDD vs. control), time (day of sample or menstrual cycle phase, see below), and group by time interactions were entered as fixed effects, subject and error were entered as random effects, and a compound symmetry covariance structure was used to model the within-subject autocorrelation among repeated measures. P values for the fixed effects are reported from the mixed model estimates; P values for group differences at specific times (and within-group differences between times) are reported from the mixed model estimates of the differences, and means and ses are shown. Calculation of the free and bound fractions of E2 was derived from the mathematical model of Sodergard et al. (19) using total E2, SHBG, and albumin concentrations.
Each subject’s menstrual cycle length was determined by the onset of menses at the beginning of the cycle sampled and the onset of menses at the end of the cycle. To facilitate analysis of grouped data from women with different menstrual cycle lengths, each woman’s menstrual cycle was divided into five phases, and phase mean values were used for analysis: 1) phase 1, the first 5 d from onset of menses (menstrual/early follicular phase); 2) phase 2, menstrual cycle d 6–2 d before ovulation (by primary ovulation date) (late follicular phase); 3) phase 3, day before, day of, and day after ovulation (ovulatory phase); 4) phase 4, 2 d after ovulation through midpoint of luteal phase (early luteal phase); and 5) phase 5, midpoint of luteal phase to onset of next menses (late luteal phase). Combined phases were then used to represent the follicular, midcycle, and luteal phases of the cycle. Final menses of the sampled hormone cycle was used to determine cycle length of that particular cycle, and individual phases were then identified.
Results
A total of 4924 women were screened for this study, with 4034 of these women telephone screened disqualified for failure of inclusion/exclusion criteria involving ethnicity, oral contraceptive usage, irregular menstrual cycles, geographic location, or taking psychotropic medication. A total of 890 subjects signed consent and were enrolled in the study for symptom monitoring and diary evaluation. One hundred twenty-nine women completed the timed biochemical and hormone evaluation. Unanticipated use of oral contraceptives or antidepressants, pregnancy, anovulatory or irregular menstrual cycles, and other calcium derangements such as primary hyperparathyroidism led to the exclusion of 14 subjects from analysis. The remaining 115 (68 women with PMDD and 47 controls) met all enrollment criteria. The demographic data and reproductive history for the overall group of 115 premenopausal women are shown in Table 1. There were no significant differences in age, age at menarche, race, height, weight, body mass index, menstrual cycle length, or pregnancies between groups. The mean age of the PMDD participant was 29.5 ± 6.1 compared with 27.9 ± 6.0 yr for the control subjects (P = 0.17). There was no difference between groups in smoking history, current smoking history, or pack years of smoking history. By definition of the inclusion criteria, the PMDD group had significantly higher luteal symptom scores than controls, as well as higher menstrual and intermenstrual scores.
Table 1.
Demographic and clinical characteristics of PMDD and control subject groups completing hormone sampling and between-group comparison
| Measure | PMDD | Control | P value |
|---|---|---|---|
| n | 68 | 47 | |
| Age (yr) | 29.5 ± 6.1 (28.0–31.0) | 27.9 ± 6.0 (26.1–29.7) | 0.17 |
| Age at menarche | 12.9 ± 1.2 (12.6–13.2) | 12.6 ± 1.2 (12.2–12.9) | 0.14 |
| No. of Caucasians (%) | 49/68 (72) | 35/47 (77) | 0.59 |
| Height (m) | 1.66 ± 0.06 (1.65–1.68) | 1.65 ± 0.07 (1.63–1.70) | 0.32 |
| Weight (kg) | 63.8 ± 13.0 (60.7–66.9) | 62.0 ± 9.3 (59.3–64.8) | 0.40 |
| BMI (kg/m2) | 23.1 ± 4.8 (21.9–24.2) | 22.5 ± 3.7 (21.4–23.6) | 0.50 |
| Years PMS symptoms | 10.8 ± 6.2 (9.3–12.3) | N/A | |
| Menstrual cycle length (d) | 28.8 ± 2.8 (28.1–29.5) | 28.4 ± 2.5 (27.6–29.1) | 0.45 |
| PMDD symptom median | |||
| Luteal phase | 21.2 ± 10.3 (18.70–23.65) | 1.0 ± 1.5 (0.60–1.49) | |
| Menstrual phase | 14.9 ± 10.5 (12.37–17.46) | 1.1 ± 1.1 (0.79–1.46) | |
| Intermenstrual phase | 1.89 ± 3.8 (0.97–2.81) | 0.3 ± 0.5 (0.13–0.45) |
Values are expressed as mean ± sd (95% confidence interval) or number (%) within group. The Student’s t test comparison of differences between-group means for continuous measures and Fisher’s exact test comparison of differences between-group proportions for categorical measures were performed. BMI, Body mass index; N/A, not applicable.
Reproductive steroid hormones–transverse means
The overall hormonal means of the PMDD and control groups are depicted in Table 2. The transverse means (overall means across the menstrual cycle) for LH, progesterone, E2, peak E2, and free E2 were not different between groups. Overall SHBG was significantly greater in the PMDD group compared with controls (61.4 ± 25.1 vs. 52.4 ± 21.3 nmol/liter; P = 0.046). Overall percent free calculated E2 was significantly greater in the asymptomatic control group compared with the PMDD group (1.50 ± 0.28 vs. 1.39 ± 0.26; P = 0.03). Calculated E2 bound to SHBG and to albumin were not significantly different between groups. However, calculated percent E2 bound to SHBG was higher in the PMDD group (45.1 ± 9.7 vs. 41.5 ± 10.3; P = 0.05), whereas calculated percent E2 bound to albumin was higher in the asymptomatic group (57.0 ± 0.1 vs. 53.5 ± 9.4; P = 0.06).
Table 2.
Reproductive steroid and hormone averages for PMDD (n = 68) and control (n = 47) subjects completing menstrual cycle serum sampling and between-group comparison
| Measure | PMDD | Control | P value |
|---|---|---|---|
| Serum LH (IU/liter) | 10.9 ± 4.0 (9.9–11.9) | 11.5 ± 5.3 (10.0–13.1) | 0.52 |
| Serum LH peak (IU/liter) | 33.5 ± 23.5 (27.8–39.2) | 34.2 ± 23.6 (27.1–41.3) | 0.29 |
| Luteal phase LH (IU/liter) | 5.7 ± 14.7 (4.2–7.2) | 7.5 ± 18.0 (5.7–9.3) | 0.064 |
| Serum SHBG (nmol/liter) | 61.4 ± 25.1 (55.4–67.5) | 52.4 ± 21.3 (46.2–58.7) | 0.046 |
| Serum albumin (g/dl) | 4.4 ± 0.2 (4.3–4.4) | 4.3 ± 0.21 (4.26–4.39) | 0.24 |
| Serum progesterone (ng/ml) | 366.2 ± 164 (326–406) | 346.4 ± 181.2 (293–400) | 0.34 |
| Luteal phase progesterone (ng/ml) | 1110 ± 769 (1034–1187) | 1143 ± 940 (1050–1235) | 0.59 |
| Serum E2 (pg/ml) | 153.2 ± 46.4 (142–164) | 150 ± 57.8 (133–167) | 0.76 |
| Serum E2 peak (pg/ml) | 276.4 ± 125 (246–306.7) | 261.0 ± 143 (218.1–303.9) | 0.55 |
| Free E2 (calculated pmol/liter) | 7.7 ± 2.4 (7.1–8.3) | 8.0 ± 2.3 (7.3–8.7) | 0.52 |
| Percent free E2 (calculated) | 1.39 ± 0.26 (1.32–1.45) | 1.50 ± 0.28 (1.41–1.58) | 0.03 |
| E2 bound to SHBG (calculated pmol/liter) | 257 ± 108 (231–283) | 239 ± 139 (199–280) | 0.45 |
| Percent E2 bound to SHBG (calculated) | 45.1 ± 9.7 (45.8- 47.5) | 41.5 ± 10.3 (38.5–44.5) | 0.05 |
| E2 bound to albumin (calculated pmol/liter) | 298 ± 91.7 (249–288) | 304 ± 86 (279–329) | 0.71 |
| Percent E2 bound to albumin (calculated) | 53.5 ± 9.4 (51.2–55.7) | 57.0 ± 10.1 (54.1–60.0) | 0.06 |
| Luteal phase E2 (pg/ml) | 156 ± 134 (141–171) | 170 ± 164 (152–189) | 0.238 |
| Luteal phase free E2 (calculated pmol/liter) | 7.6 ± 7.0 (6.8–8.3) | 8.9 ± 8.4 (8.0–9.8) | 0.032 |
| Luteal phase percent free E2 (calculated) | 1.35 ± 0.35 (1.28–1.41) | 1.47 ± 0.43 (1.39–1.55) | 0.018 |
Values are expressed as mean ± sd (95% confidence interval). The Student’s t test comparison of differences between-group means was performed. To convert E2 pg/ml to pmol/liter, multiply by 3.67.
Mixed linear model analysis with fixed effects for group, time, and group by time interaction showed that the ovarian steroid hormones varied significantly across the menstrual cycle in both groups of subjects with ovulatory cycles. E2, progesterone, and LH changed significantly over the menstrual cycle phases: for E2, F(4, 404) = 129.1, P < 0.001; for progesterone, F(4, 415) = 235.6, P < 0.001; and for LH, F(4, 415) = 105.2, P < 0.001). SHBG showed a significant main effect of phase (F(4, 397) = 12.5; P < 0.001) and a significant effect of group (F(1, 113) = 4.323; P = 0.040). Free E2 showed a significant effect of phase (F(4, 409) = 133.1; P < 0.001). Percent free E2 showed a significant effect of group (F(1, 115) = 5.15; P = 0.025) and phase (F(4, 397) = 16.5; P < 0.001).
Reproductive hormones–phase means
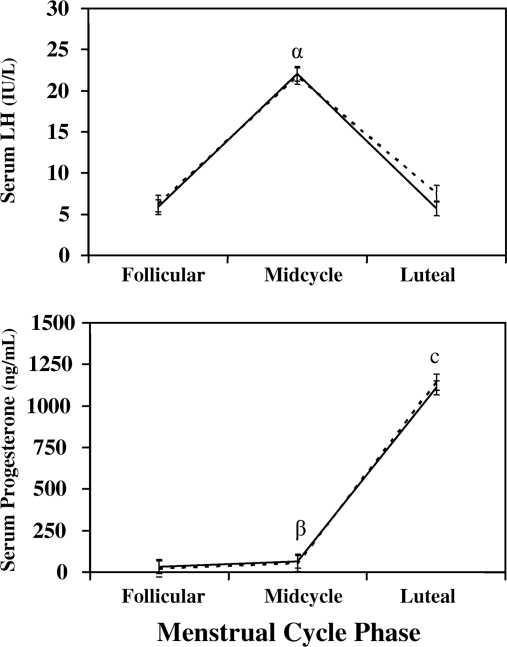
Between-group comparisons of the different phases of the menstrual cycle (Fig. 1) demonstrated that the LH concentrations were not significantly different during the individual follicular or midcycle phases of the menstrual cycle. However, luteal phase LH concentrations were greater in the asymptomatic control group (LH 7.5 ± 17.9 vs. 5.7 ± 14.7; P = 0.13) compared with the PMDD group.
Figure 1.
Serum LH and progesterone means for PMDD (solid line) and control subjects (dotted line) at the follicular phase, midcycle, and luteal phases of the menstrual cycle (means and se). Significant differences are shown for between-group differences and within-group phase differences. The within-group comparison in LH for the midcycle compared with follicular and luteal phases for both the PMDD and control groups with a P value less than 0.05 (α). A within-group comparison in midcycle progesterone compared with follicular phase for the PMDD and control groups with a P value less than 0.05 (β).Within-group comparisons in luteal progesterone for both PMDD and control groups compared with follicular and midcycle phases with a P value less than 0.05 (c). LH is in IU/liter (normal range 0.5–76.3), and progesterone is in ng/ml (normal range 50–2500).
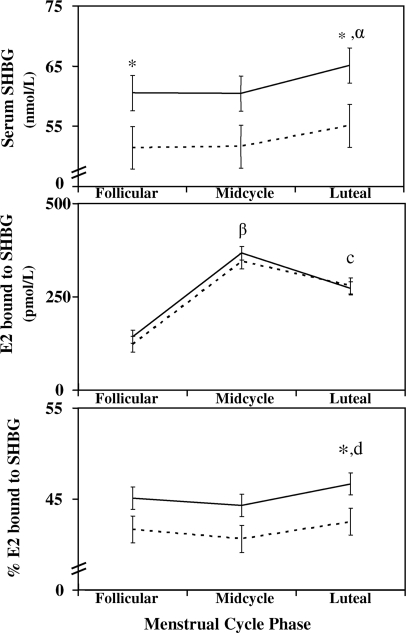
SHBG was significantly higher in the PMDD group (Fig. 2) for both follicular and luteal phases, with a trend toward significance at midcycle (follicular phase 60.5 ± 31.7 vs. 51.4 ± 38.2 nmol/liter, P = 0.047; luteal phase 65.1 ± 32.3 vs. 55.1 ± 38.9 nmol/liter, P = 0.03; and midcycle phase 60.4 ± 33.9 vs. 51.7 ± 40.8, P = 0.06). In both groups, SHBG significantly increased from the follicular to luteal phase (PMDD 60.5 ± 31.7 vs. 65.1 ± 32.3 nmol/liter, P < 0.001; controls 51.4 ± 38.2 vs. 55.1 ± 38.9 nmol/liter, P = 0.002).
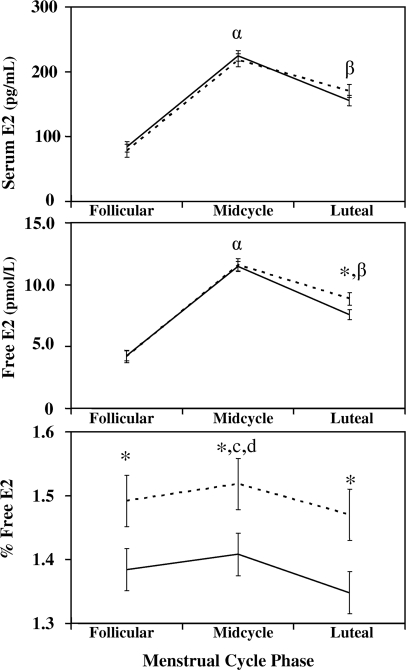
Figure 2.
Serum E2, free E2, and percent free E2 means for PMDD (solid line) and control subjects (dotted line) at the follicular phase, midcycle, and luteal phases of the menstrual cycle (means and se). Significant differences are shown for between-group differences and within-group phase differences. An asterisk (*) above phase means indicates a between-group comparison difference with a P value less than 0.05 at that phase. Within-group comparisons in midcycle E2 and free E2 for PMDD and control groups compared with follicular and luteal phases with a P value less than 0.05 (α). Within-group comparisons in luteal E2 and free E2 for PMDD and control groups compared with follicular phase with a P value less than 0.05 (β). Within-group comparisons in midcycle percent free E2 for the PMDD group compared with follicular and luteal phases with a P value less than 0.05 (c). A within-group comparison in midcycle percent free E2 for the control group compared with luteal phase with a P value less than 0.05 (d). E2 is in pg/ml (normal range 20–750).
Total E2 and progesterone concentrations were not different between groups during the individual phases of the menstrual cycle (Fig. 1). Free E2 was significantly lower in the PMDD group compared with controls during the luteal phase (PMDD 7.6 ± 7.0 vs. control 8.9 ± 8.4 pmol/liter; P = 0.032; Fig. 2). Throughout all phases of the menstrual cycle, percent free E2 was significantly lower in the PMDD group compared with controls (follicular phase 1.384 ± 0.348 vs. 1.492 ± 0.422, P = 0.033; midcycle phase 1.408 ± 0.372 vs. 1.518 ± 0.451, P = 0.034; and luteal phase 1.348 ± 0.351 vs. 1.470 ± 0.427, P = 0.018). Although calculated E2 bound to SHBG was not different between groups during the luteal phase, E2 bound to albumin was significantly greater in the control group during this same period (337 ± 323 vs. 293 ± 264 pmol/liter; P = 0.045). Percent E2 bound to SHBG and bound to albumin were significantly different between groups. Percent E2 bound to SHBG was greater in all phases of the menstrual cycle for the PMDD group compared with controls, with a significant difference during the luteal phase (follicular phase 45.2 ± 13.2 vs. 41.6 ± 15.8, P = 0.06; midcycle 44.3 ± 14.0 vs. 40.6 ± 16.8, P = 0.06; and luteal phase 46.7 ± 13.4 vs. 42.5 ± 16.1, P = 0.03; Fig. 3). In contrast, percent E2 bound to albumin was significantly greater for controls during the luteal phase compared with the PMDD group (follicular phase 56.9 ± 15.4 vs. 53.4 ± 12.8, P = 0.06; midcycle 57.9 ± 16.4 vs. 54.3 ± 13.6, P = 0.06; and luteal phase 56.0 ± 15.7 vs. 52.0 ± 13.1, P = 0.03). Within-group comparisons between phases demonstrated the well-known fluctuations among the various menstrual cycle phases in both groups for LH, E2, free E2, percent free E2, and progesterone (Figs. 1–3).
Figure 3.
Serum SHBG, SHBG-bound E2, percent SHBG-bound E2 means for PMDD (solid line) and control subjects (dotted line) at the follicular phase, midcycle, and luteal phases of the menstrual cycle (means and se). An asterisk (*) above phase means indicates a between-group comparison difference with a P value less than 0.05 at that phase. A within-group comparison in luteal SHBG for PMDD and control groups compared with midcycle and follicular phases with a P value less than 0.05 (α).Within-group comparisons in luteal SHBG-bound E2 for PMDD and control groups compared with follicular and luteal phases with a P value less than 0.05 (β). Within-group comparisons in luteal SHBG-bound E2 for PMDD and control groups compared with follicular phase with a P value less than 0.05 (c). Within-group comparison in luteal percent SHBG-bound E2 for the PMDD and control groups compared with midcycle with a P value less than 0.05 (d). SHBG is in nmol/liter (normal range 17–120 nmol/liter).
Discussion
PMDD, also described as severe PMS, is a luteal phase disorder characterized by the cyclical recurrence of mood and physical symptoms during the latter half of the menstrual cycle. To date, the underlying pathophysiology of this phase-related disorder has remained unexplained (20,21). This investigation that systematically screened a large number of potential candidates for the distinct PMDD and control populations has found that luteal phase concentrations of free E2, percent free E2, and SHBG differ significantly between women with and without PMDD. Both free E2 and percent free E2 were significantly lower, and SHBG concentrations significantly higher in PMDD subjects compared with asymptomatic controls. These differences in percent free E2 and SHBG between groups were found throughout the menstrual cycle but became clinically relevant in the PMDD group with the statistically significant lower concentrations of free E2 during the luteal phase of the cycle. No luteal phase differences were noted in progesterone concentrations.
Estrogen, although known to be important in both ovulation and reproduction, has been recognized to enhance well-being with major effects on the central nervous system in areas other than the hypothalamus (22,23). In this regard, estrogen appears to exert actions in areas of the brain that are important for learning and memory, emotions and affective state, and pain sensitivity. It has widespread regulatory effects throughout the brain, including the brainstem and midbrain catecholaminergic neurons, midbrain serotonergic pathways, midbrain dopaminergic activity, and the basal forebrain cholinergic system (24). Evidence suggests that estrogen modulates the metabolism of monoamine and neuropeptide transmitter pathways, and may affect those mechanisms involved in mood and affective disorders (25,26). Estrogen stimulates a significant increase in dopamine (D2) receptors in the striatum of the brain, and an increase in the density of 5-hydroxytryptamine (5-HT2A) binding sites in the anterior frontal, cingulate, and primary olfactory cortex of the brain (14), areas concerned with mood and behavior. In one investigation, a single pulse of E2 in female rats induced a significant increase in the density of 5-HT2A receptors in the forebrain (27). In another investigation, serotonin 5-HT2A receptor binding in the prefrontal cortex of the brain as assessed by positron emission tomography was significantly increased in 10 postmenopausal women after estrogen therapy. Acute changes in ovarian function or even relatively lower estrogen concentrations during the luteal phase of the menstrual cycle may influence serotonergic metabolism and neurotransmitter reuptake, both thought to mediate mood and emotion (28).
Over the years, different hypotheses involving the ovarian steroid hormones have been proposed to explain the luteal phase occurrence of severe PMS symptoms. Most investigations to date have not substantiated consistent differences in the concentrations of either E2 or progesterone between symptomatic or asymptomatic women. Explanations for normal serum hormone levels have even included the possibility of variations in the estrogen receptor with genotypical differences likely to mediate differential behavioral responses. A recent investigation by Huo et al. (29) has now demonstrated preliminary evidence for this hypothesis with four single nucleotide polymorphisms in intron of estrogen receptor 1 found to be significantly different in PMDD and control subjects.
Our investigation with careful subject selection and well-matched controls is the largest study of women with and without PMDD in which multiple-timed measures of hormones were obtained across the menstrual cycle. Although we did not find significant differences in overall serum E2 or luteal phase E2 concentrations, free E2, percent free E2, and SHBG were found to differ significantly between women. Differences in free E2 or bioavailable E2 rather than total E2 may help explain the myriad of PMDD symptoms, such as depression, moodiness, and irritability. Our findings provide support to the hypothesis that a relative estrogen deficiency may have a role in the pathogenesis of PMS. These findings are similar in part to those demonstrating lower serum E2 levels and diminished urinary E2 excretion during the luteal phase in those with premenstrual symptoms, and may also help explain the beneficial effect of E2 therapy administered in PMS (12,30,31). In contrast, our data differ from the findings of Wang et al. (10), who reported higher E2 and lower progesterone concentrations during the luteal phase in 12 women with PMS compared with controls, and from the data of Seippel and Backstrom (11), who related higher luteal phase-E2 concentrations with greater PMS symptom severity. Both of these studies were relatively small, and neither free E2 nor SHBG was measured. It is unclear why so many studies have reported different results. Methodological limitations may exist as to the definition of what is a true asymptomatic control, actual sample size, and to issues related to menstrual cycle length. The extreme variability of menstrual cycle length and failure to properly align the individual cycle phases may be the single most important confounder in conflicting PMDD data.
Along the same line, but counter to the positive treatment one would have anticipated on E2 therapy, another investigation demonstrated that administration of higher doses of E2 during the luteal phase in women with PMS worsened PMS symptomatology, and resulted in more negative mood symptoms compared with placebo (32). The reported contradictory effects of E2 on luteal phase symptoms are still not fully explained. E2 may be influencing another hormonal axis such as the vitamin D pathway, resulting in disparate symptomatic responses. A previous investigation by our group (15) demonstrated lower 1,25 dihydroxyvitamin D concentrations during the luteal phase in women with PMDD compared with controls. Lower E2 concentrations during the luteal phase, as noted in this study, may adversely affect vitamin D synthesis, resulting lower in 1,25 dihydroxyvitamin D concentrations with diminished calcium access and reserves.
SHBG is a glycoprotein synthesized in the liver, which transports and regulates the access of sex steroids to their target tissues (33). Levels of SHBG may be influenced by nutrition, sex steroids, thyroid hormones, peptide hormones as insulin, and genetics (34). In vitro data suggest that both androgens and estrogens stimulate SHBG production; however, in vivo androgens appear to suppress SHBG, whereas estrogens are associated with elevated levels. One study investigated the effect of various growth factors on SHBG production in the human hepatoblastoma cell line Hep G2. Hep G2 cell cultures were incubated with IGF-I, epidermal growth factor, and TGF (35). All these growth factors resulted in a significant decrease in SHBG production in cell culture medium. Our study found SHBG to be significantly different between the two study cohorts across the menstrual cycle, with higher concentrations in the PMDD group compared with controls, particularly during the luteal phase. Interestingly, we have also recently found in this same study cohort that IGF-I concentrations are significantly different as well, with lower concentrations in PMDD subjects compared with controls (36). Our clinical observations of an inverse relationship of IGF-I to SHBG concentrations appear to support the work of Plymate et al. (35), in which growth factors such as IGF-I may regulate SHBG. Higher SHBG concentrations as noted in the PMDD group may limit the bioavailability of E2 to the brain, liver, and other tissues, and ultimately affect mood.
In this investigation, subjects were limited to Caucasian and Hispanic women. In addition, funding and feasibility constraints limited hormonal sampling to 8 d of the menstrual cycle, and hormonal events may have been missed. Free E2 was calculated and not measured in this study. Although methods used to calculate free E2 have been used in many epidemiological and clinical investigations, calculated E2 is not the same as measured E2.
In conclusion, differences in the concentrations of free E2, percent free E2, and SHBG were demonstrated across the menstrual cycle in women with PMDD compared with asymptomatic controls. The differences in the concentrations of SHBG and free E2 between women with and without luteal phase symptoms may partially elucidate the existing controversy on the connection between ovarian steroid hormones and luteal phase symptoms.
Footnotes
This work was supported by the National Institute of Health-National Institute of Diabetes and Digestive and Kidney Diseases Grant DK57869-03 in conjunction with National Institute of Mental Health and Institute of Women’s Health.
Disclosure Statement: The authors have nothing to disclose.
First Published Online October 23, 2007
Abbreviations: E2, Estradiol; 5-HT2A, 5-hydroxytryptamine; PMDD, premenstrual dysphoric disorder; PMS, premenstrual syndrome.
References
- Rapkin A 1992 The role of serotonin in premenstrual syndrome. Clin Obstet Gynecol 35:629–636 [DOI] [PubMed] [Google Scholar]
- Reid RL, Yen SS 1981 Premenstrual syndrome. Am J Obstet Gynecol 139:85–104 [DOI] [PubMed] [Google Scholar]
- Steiner M 1992 Female specific mood disorders. Clin Obstet Gynecol 35:599–611 [DOI] [PubMed] [Google Scholar]
- Rubinow DR 1992 The premenstrual syndrome: new views. JAMA 268:1908–1912 [PubMed] [Google Scholar]
- Casson P, Haln PM, Van Vugt DA, Reid RL 1990 Lasting response to ovariectomy in severe intractable premenstrual syndrome. Am J Obstet Gynecol 162:99–105 [DOI] [PubMed] [Google Scholar]
- Graham CA, Sherwin BB 1992 A prospective treatment study of premenstrual symptoms using a triphasic oral contraceptive. J Psychosom Res 36:357–366 [DOI] [PubMed] [Google Scholar]
- Brown C, Ling F, Andersen R, Farmer R, Arheart K 1994 Efficacy of depot leuprolide in premenstrual syndrome: effect of symptoms severity and type in a controlled trial. Obstet Gynecol 84:779–786 [PubMed] [Google Scholar]
- Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR 1998 Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med 338:209–216 [DOI] [PubMed] [Google Scholar]
- Rubinow D, Hoban C, Grover GN, Galloway DS, Roy-Byrne P, Andersen R, Merriam G 1988 Changes in plasma hormones across the menstrual cycle in patients with menstrually related mood disorder and in control subjects. Am J Obstet Gynecol 158:5–11 [DOI] [PubMed] [Google Scholar]
- Wang M, Seippel L, Purdy R, Backstrom T 1996 Relationship between symptom severity and steroid variation in women with premenstrual syndrome. J Clin Endocrinol Metab 81:1076–1082 [DOI] [PubMed] [Google Scholar]
- Seippel L, Backstrom T 1998 Luteal phase estradiol relates to symptom severity in patients with premenstrual syndrome. J Clin Endocrinol Metab 83:1988–1992 [DOI] [PubMed] [Google Scholar]
- Blum I, Lerman M, Misrachi I, Nordenberg Y, Grosskopf I, Wewizman A, Levy-Schiff R, Sulkes J, Vered Y 2004 Lack of plasma norepinephrine cyclicity, increased estradiol during the follicular phase, and of progesterone and gonadotropins at ovulation in women with premenstrual syndrome. Neuropsychobiology 50:10–15 [DOI] [PubMed] [Google Scholar]
- Hsiao CC, Liu CY, Hsiao MC 2004 No correlation of depression and anxiety to plasma estrogen and progesterone levels in patients with premenstrual dysphoric disorder. Psychiatry Clin Neurosci 58:593–599 [DOI] [PubMed] [Google Scholar]
- Fink G, Sumner BE, Rosie R, Grace O, Quinn JP 1996 Estrogen control of central neurotransmission: effect on mood, mental state and memory. Cell Mol Neurobiol 16:325–344 [DOI] [PubMed] [Google Scholar]
- Thys-Jacobs S, McMahon D, Bilezikian JP 2007 Cyclical changes in calcium metabolism across the menstrual cycle in women with premenstrual dysphoric disorder. J Clin Endocrinol Metab 92:2952–2959 [DOI] [PubMed] [Google Scholar]
- Task Force on DSM-IV 1994 Premenstrual dysphoric disorder. In: Diagnostic and statistical manual of mental disorders: DSM-IV. 4th ed. Washington, DC: American Psychiatric Association [Google Scholar]
- Spitizer RL, Williams JBW, Gibbon M, First MB 1992 The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry 49:624–629 [DOI] [PubMed] [Google Scholar]
- Alvir JMJ, Thys-Jacobs S 1991 Premenstrual and menstrual symptom clusters and response to calcium treatment. Psychopharmacol Bull 27:145–148 [PubMed] [Google Scholar]
- Sodergard R, Backstrom T, Shanbhag V, Carstensen H 1982 Calculation of free and bound fractions of testosterone and estradiol-17B to human plasma proteins at body temperature. J Steroid Biochem 16:801–810 [DOI] [PubMed] [Google Scholar]
- Cronje WH, Vashisht A, Studd JWW 2004 Hysterectomy and bilateral oophorectomy for severe premenstrual syndrome. Hum Reprod 19:2152–2155 [DOI] [PubMed] [Google Scholar]
- Yonkers K, Brown C, Pearlstein T, Foegh M, Sampson-Landers C, Rapkin A 2005 Efficacy of a new low dose oral contraceptive with drospirenone in premenstrual dysphoric disorder. Obstet Gynecol 106:492–501 [DOI] [PubMed] [Google Scholar]
- Wiklund I, Karlberg J, Mattson LA 1993 Quality of life of postmenopausal women on a regimen of transdermal estradiol therapy: a double-blind placebo-controlled study. Am J Obstet Gynecol 168(3 Pt 1):824–830 [DOI] [PubMed] [Google Scholar]
- Joffe H, Cohen LS 1998 Estrogen, serotonin and mood disturbance: where is the therapeutic bridge? Biol Psychiatry 44:798–811 [DOI] [PubMed] [Google Scholar]
- McEwen B 1999 Molecular and neuroanatomical basis for estrogen effects in the central nervous system. J Clin Endocrinol Metab 84:1790–1797 [DOI] [PubMed] [Google Scholar]
- Wagner EJ, Manzanares J, Moore KE, Lookingland KJ 1994 Neurochemical evidence that estrogen induced suppression of kappa-opioid receptor mediated regulation of tuberoinfundibular dopaminergic neurons is prolactin independent. Neuroendocrinology 59:197–201 [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Qui J, Wagner EJ, Ronnekleiv O 2003 Rapid effects of estrogen on G protein coupled receptor activation of potassium channels in the central nervous system. J Steroid Biochem Mol Biol 83:187–193 [DOI] [PubMed] [Google Scholar]
- Summer BE, Fink G 1995 Estrogen increases the density of 5-hydroxytryptamine (2A) receptors in cerebral cortex and nucleus accumbens in the female rat. J Steroid Biochem Mol Biol 54:15–20 [DOI] [PubMed] [Google Scholar]
- Moses EL, Dreverts WC, Smith G, Mathis CA, Kairo BN, Butters MA, Leondires MP, Greer PJ, Lopresti B, Loucks TL, Berga SL 2000 Effects of estradiol and progesterone administration on human serotonin 2A receptor binding: a PET study. Biol Psychiatry 48:854–860 [DOI] [PubMed] [Google Scholar]
- Huo L, Straub RE, Roca C, Schmidt PJ, Shi K, Vakkalanka R, Weinberger DR, Rubinow DR 2007 Risk for premenstrual dysphoric disorder is associated with genetic variation in ESR1, the estrogen receptor alpha gene. Biol Psychiatry 62:925–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods NF, Lentz MJ, Mitchell ES, Shaver J, Heitkemper M 1998 Luteal phase ovarian steroids, stress arousal, premenses, perceived stress and premenstrual symptoms. Res Nurs Health 21:129–142 [DOI] [PubMed] [Google Scholar]
- Steiner M, Steinberg S, Stewart D, Carter D, Berger C, Reid R, Grover D, Streiner D 1995 Fluoxetine in the treatment of premenstrual dysphoria. N Engl J Med 332:1529–1534 [DOI] [PubMed] [Google Scholar]
- Dhar V, Murphy BE 1990 Double blind randomized crossover trial of luteal phase estrogens (Premarin) in the premenstrual syndrome. Psychoneuroendocrinology. 15:489–493 [DOI] [PubMed] [Google Scholar]
- Hammond GL 1990 Molecular properties of corticosteroid binding globulin and the sex steroid binding proteins. Endocr Rev 11:65–79 [DOI] [PubMed] [Google Scholar]
- Siiteri PK, Murai JT, Hammond GL, Nisker JA, Raymoure WJ, Kuhn RW 1982 The serum transport of steroid hormones. Recent Prog Horm Res 38:457–510 [DOI] [PubMed] [Google Scholar]
- Plymate SR, Hoop RC, Jones RE, Matej LA 1990 Regulation of sex hormone-binding globulin production by growth factors. Metabolism 39:967–970 [DOI] [PubMed] [Google Scholar]
- Thys-Jacobs S, McMahon D, Bilezikian JP, Lower IGF-1 concentrations in women with premenstrual dysphoric disorder. Am J Obstet Gyn, in press [DOI] [PubMed] [Google Scholar]