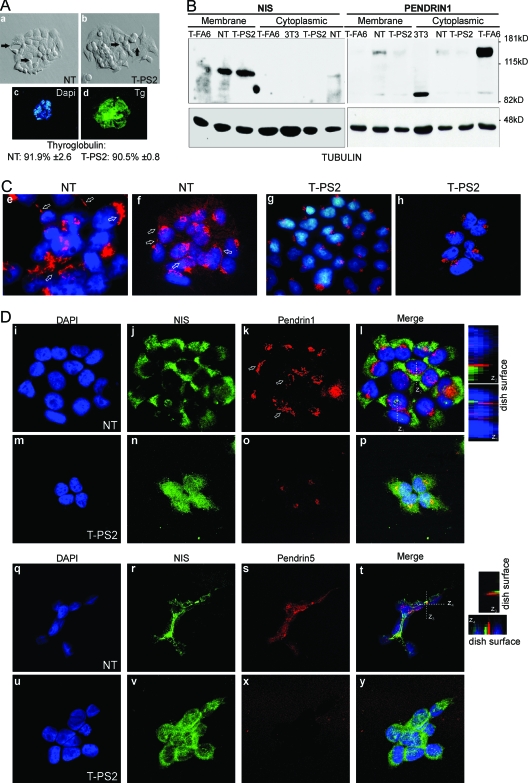
Figure 4.
At the outer plasma membrane, normal thyrocytes (NT) express fully glycosylated NIS and pendrin, whereas cells from the propositus of family B (T-PS2) express only NIS. A, The appearance of cultured NT (a) and T-PS2 cells (b) is similar under the phase-contrast microscope. The cells are small and polygonal and leave round spaces between them, recalling a follicular structure (see arrows). In both cell lines, using DAPI for nuclear counterstaining, practically all cells expressed Tg (shown for T-PS2, c and d). B, Western blotting against NIS (left) and pendrin (right) using hot SDS extracts enriched in plasma membrane proteins (membrane) or 1% Triton extracts with intracellular membrane content, i.e. Golgi or ER (cytoplasmic). As a loading control, the membranes were rehybridized against tubulin. NIS is expressed mainly as the 100-kDa fully glycosylated form at the plasma membrane in NT, T-PS2, and the T-FA6 primary-culture follicular adenoma line from our BANTTIC collection; smaller bands around 80 kDa correspond to nonglycosylated immature NIS. Intracellular levels are undetectable in all lines except NT, in which a faint band can be seen suggesting slightly greater NIS expression. Mouse 3T3 fibroblasts were used as negative control. NT cells express the 130-kDa fully glycosylated pendrin at the plasma membrane, but only a very weak band (less than 5%) can be seen in T-PS2 and T-FA6 cells. The faint 85-kDa band is the nonglycosylated protein. In intracellular membranes, NT cells express a weak band corresponding to pendrin in the process of sorting, and T-PS2 cells likewise show only a weak band. However, T-FA6 cells retain pendrin intracellularly. C, Confocal immunofluorescence images using the same pendrin antibody as in B, PS1Ab (specific for the N-terminal part of the protein). DAPI is used to show nuclei. Both a ×40 water-immersion objective with ×3 magnification (e and g) and a ×63 oil-immersion objective with a ×1.5 magnification (f and h) were used. PS1Ab staining is localized in the Golgi (round spots beside the nuclei) and the plasma membrane (straight lines indicated by arrows) in normal thyrocytes (NT); in T-PS2 thyrocytes, pendrin appears to be retained in the Golgi (truncated proteins). D, Double immunofluorescence with NIS and PS1Ab shows intracellular and plasma membrane staining (arrows) of both proteins in NT (i–l, ×1000). Note that membrane colocalization is not frequent: the z projections obtained through stacking of confocal images show that, although localized in the membrane, the two proteins rarely coincide in the same locations, as indicated by the scarce yellow spots. T-PS2 cells (m–p, ×1000) show intracellular and plasma membrane staining of NIS, but only one isolated spot of pendrin can be seen at the plasma membrane, whereas the rest is retained in the Golgi. Similar double-immunofluorescence studies were performed using PS5Ab, specific for the C-terminal end of the pendrin protein. Both NIS and pendrin are localized at the plasma membrane in NT cells (q–t, ×1000), although both proteins seem to occupy different membrane domains (see z projections). In T-PS2 cells, the PS5Ab image is overexposed to demonstrate the absence of specific staining (u–y).