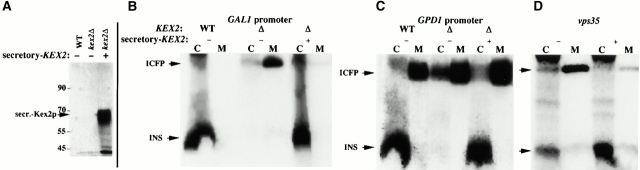
Figure 9.
Replacement of Kex2p by secretory Kex2p is sufficient to cause intracellular insulin retention. The strains are pep4Δ mutants in order to stabilize the intracellular protein. Cells were pulse labeled for 30 min and chased for 60 min. (A) Chase media bathing the indicated strains were collected and immunoprecipitated with an antibody against Kex2p. (B) Intracellularly retained and secreted forms of immunoreactive insulin upon ICFP expression with a GAL1 promoter on a centromeric plasmid. Note that when truncated secretory Kex2p is expressed in the kex2Δ mutant it alters protein targeting to the extent that insulin is made. (C) Intracellularly retained and secreted forms of immunoreactive insulin upon ICFP expression with a GPD1 promoter on a centromeric plasmid. Note that at the higher levels of ICFP expression ICFP endoproteolytic processing is incomplete and the unprocessed portion is secreted. (D) Intracellularly retained and secreted forms of immunoreactive insulin upon ICFP expression with a GAL1 promoter in vps35 cells. In these cells as well, secretory Kex2p expression alters the protein targeting to the extent that insulin is made.