Figure 1.
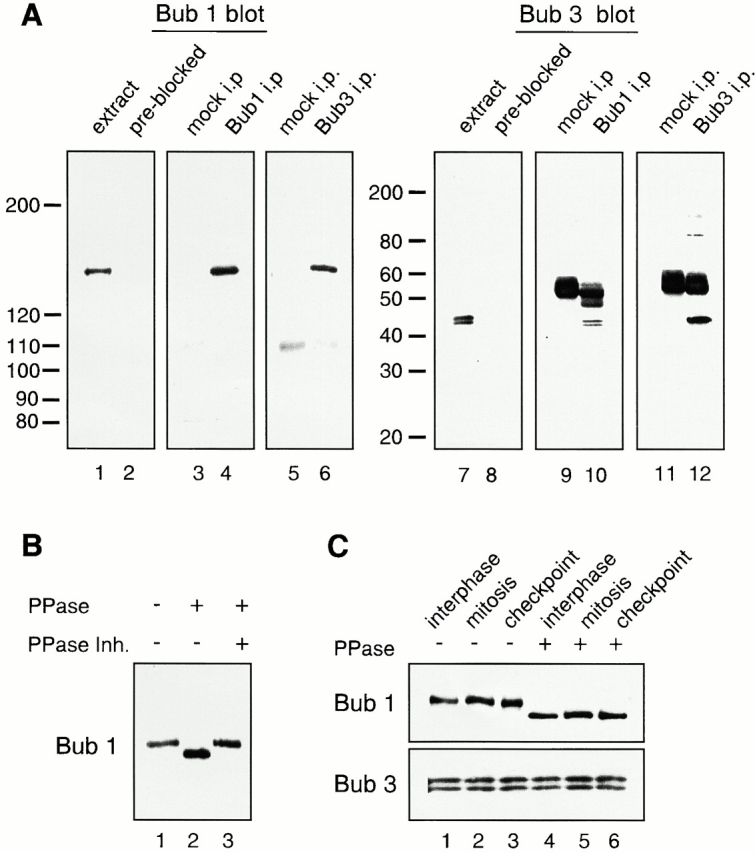
Analysis of Bub1 and Bub3 from Xenopus egg extracts. (A) Characterization of affinity-purified anti-Bub1 and anti-Bub3 antibodies. CSF-arrested egg extracts (lanes 1, 2, 7, and 8), or immunoprecipitates generated with control IgG (lanes 3, 5, 9, and 11), with anti-Bub1 (lanes 4 and 10) or anti-Bub3 antibodies (lanes 6 and 12) were probed with affinity-purified anti-Bub1 antibodies (lanes 1, 3–6), anti-Bub1 antibodies preblocked with recombinant Bub1 protein (lane 2), anti-Bub3 antibodies (lanes 7, 9–12), or anti-Bub3 preblocked with recombinant Bub3 protein (lane 8). The migration of molecular weight standards is indicated on the left. The prominent 55-kD protein is the IgG heavy chain. (B) Bub1 is a phosphoprotein. Bub1 was immunoprecipitated from CSF-arrested extract and treated with LPP (PPase) in the presence or absence of phosphatase inhibitors (PPase Inh.) as indicated on the top. (C) Bub1 is phosphorylated at interphase and mitosis, and under the checkpoint-active condition. Bub1 and Bub3 were coimmunoprecipitated with anti-Bub1 antibodies from interphase (lanes 1 and 4), CSF-arrested (lanes 2 and 5, mitosis), or checkpoint-active extracts (lanes 3 and 6, checkpoint), and left untreated (lanes 1–3) or treated with LPP (lanes 4–6). The upper panel was probed with anti-Bub1 antibody and the lower panel was probed with anti-Bub3 antibody.
