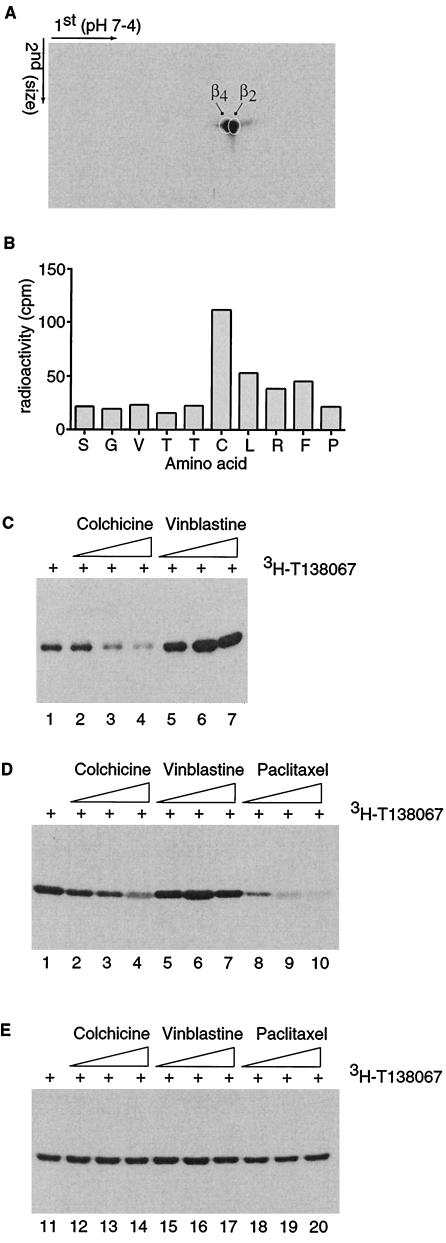
Figure 2.
Selective binding of T138067 to Cys-239 of β2- and β4-tubulin. (A) Shown is an autoradiogram of a 3H-T138067-labeled cell extract derived from MCF7/ADR cells and separated by two dimensional gel electrophoresis. The first and second dimensions of the gel electrophoresis are shown. The positions of the individual β-tubulin isotypes are indicated. (B) Cys-239 of β2-tubulin bound by 3H-T138067. The 3H-T138067-modified tubulin peptide isolated from a cyanogen bromide digest of tubulin was subjected to Edman degradation. The first 10 aa corresponding to peptide Ser-234 to Met-267 of β2-tubulin and the radioactivity (cpm) coeluting with each of the amino acids (in standard single-letter code) are shown. (C) Colchicine competes for binding of T138067 to Cys-239 in vitro. Purified brain tubulin (5 μM) was incubated in the absence of drug (lane 1) or pretreated for 2 hr with colchicine [lane 2 (0.5 μM), lane 3 (5 μM), and lane 4 (25 μM)] or vinblastine [lane 5 (0.5 μM), lane 6 (5 μM), and lane 7 (25 μM)] before 5 μM of 3H-T138067 was added to the reaction for 60 min. (D and E) Colchicine competes for binding of T138067 to Cys-239 in MCF7 (D), but not in MCF7/ADR (E) cells. Cells were incubated in the absence of drug (lanes 1 and 11) or pretreated for 24 hr with colchicine [lanes 2 and 12 (0.5 μM), lanes 3 and 13 (1 μM), lanes 4 and 14 (1.5 μM)], vinblastine [lanes 5 and 15 (0.5 μM), lanes 6 and 16 (1 μM), and lanes 7 and 17 (1.5 μM)], or paclitaxel [lanes 8 and 18 (0.5 μM), lanes 9 and 19 (1 μM), and lanes 10 and 20 (1.5 μM)] before cells were exposed to 400 nM 3H-T138067 for 3 hr.