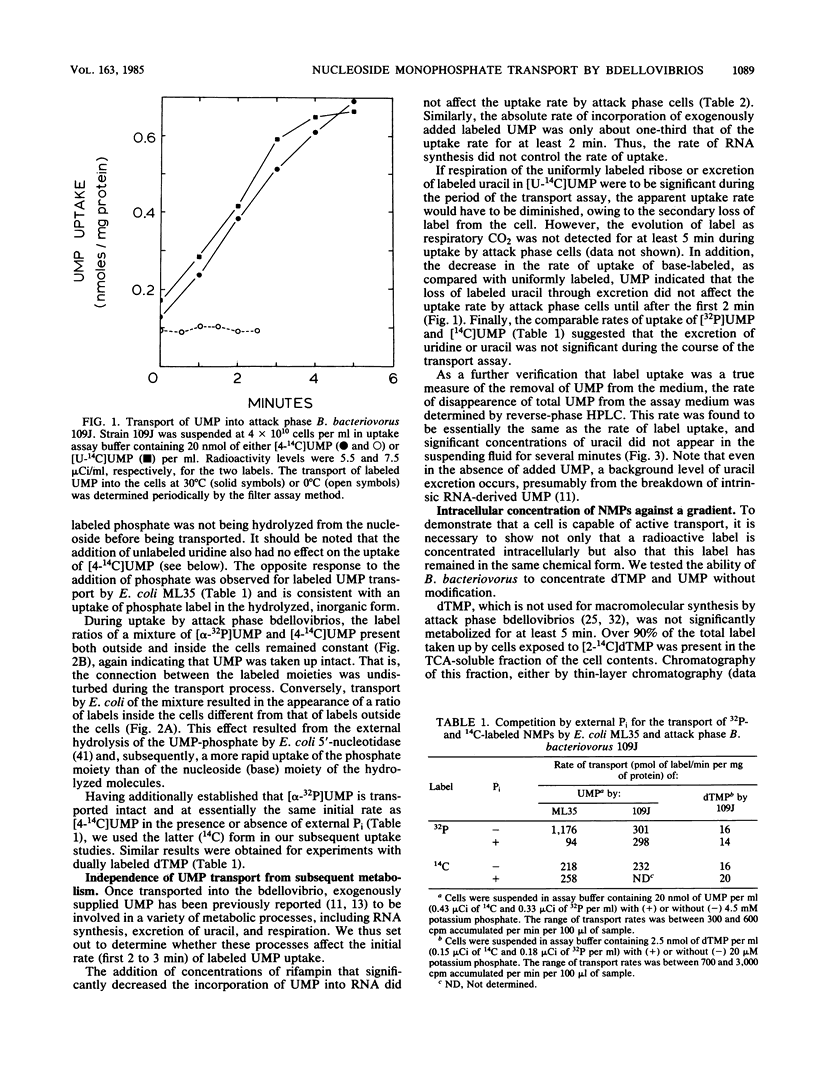
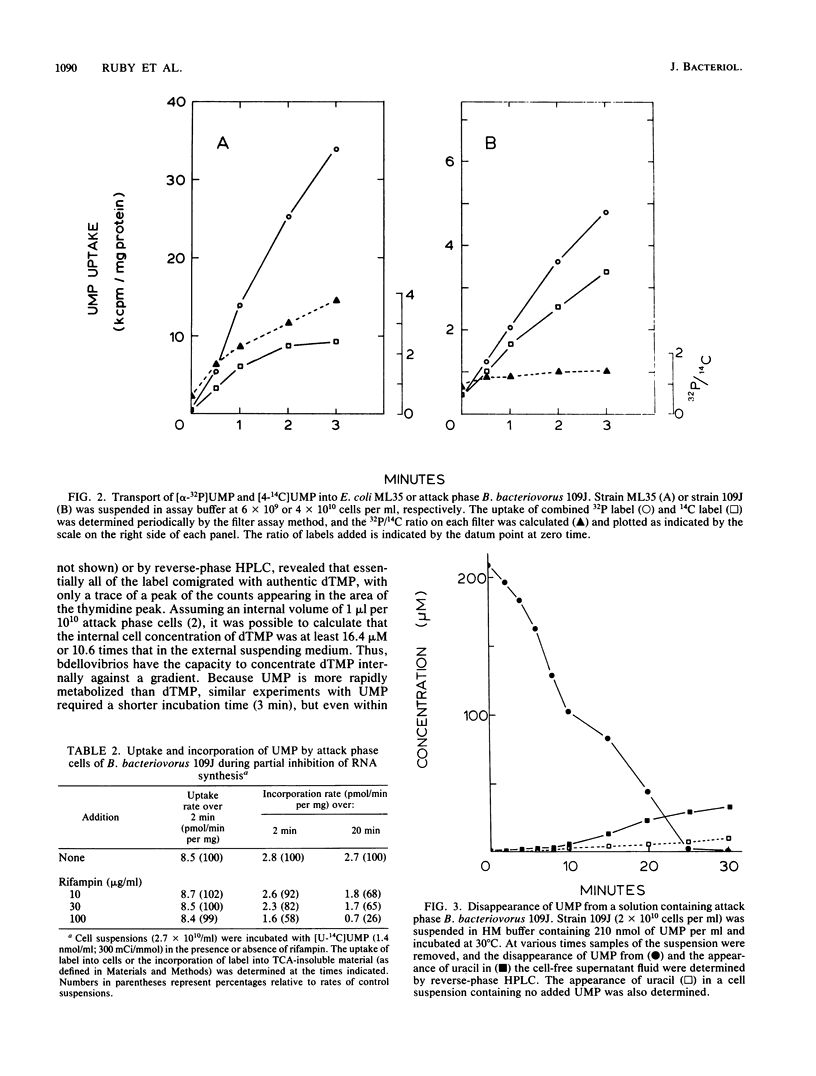
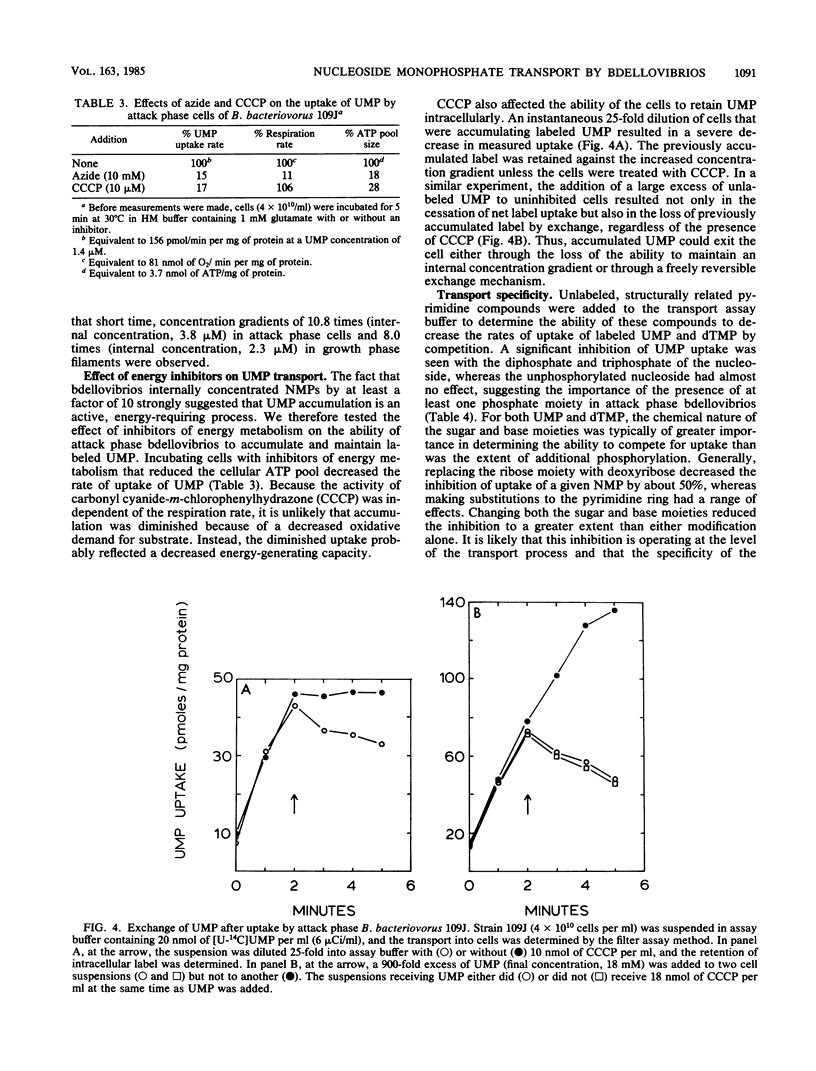
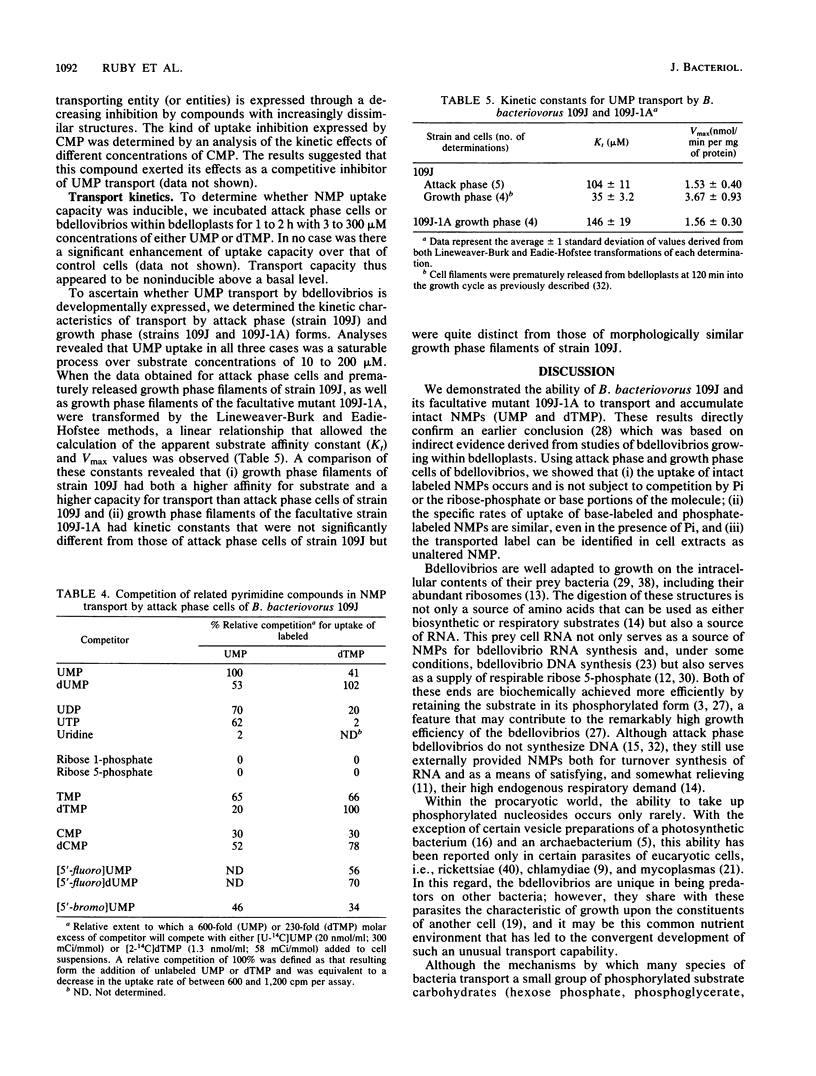
Abstract
The degraded nucleic acids and ribosomes of its prey cell provide Bdellovibrio bacteriovorus 109J with a source of ribonucleoside monophosphates and deoxyribonucleoside monophosphates for biosynthesis and respiration. We demonstrate that bdellovibrios, in contrast to almost all other bacteria, take up these nucleoside monophosphates into the cell in an intact, phosphorylated form. In this way they are able to assimilate more effectively the cellular contents of their prey. Studies with UMP and dTMP demonstrate that they are transported and accumulated against a concentration gradient, achieving internal levels at least 10 times the external levels. Treatment of the bdellovibrios with azide or carbonyl cyanide m-chlorophenylhydrazone eliminates their ability to either transport or maintain accumulated UMP and suggests the presence of a freely reversible exchange mechanism. There are at least two separate classes of transport systems for nucleoside monophosphates, each exhibiting partial specificity for either ribonucleoside monophosphates or deoxyribonucleoside monophosphates. Kinetic analyses of UMP transport in different developmental stages of strain 109J indicate that each stage expresses a single, saturable uptake system with a distinct apparent substrate affinity constant (Kt) of 104 microM in attack phase cells and 35 microM in prematurely released growth phase filaments. The capacity for transport of UMP by the growth phase filaments was 2.4 times that of the attack phase cells. These data, in addition to the apparent lack of environmental control of UMP transport capacity in attack phase cells, suggest that there are two transport systems for UMP in bdellovibrios and that the high-affinity, high-capacity growth phase system is developmentally regulated.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaman T. C., Hitchins A. D., Ochi K., Vasantha N., Endo T., Freese E. Specificity and control of uptake of purines and other compounds in Bacillus subtilis. J Bacteriol. 1983 Dec;156(3):1107–1117. doi: 10.1128/jb.156.3.1107-1117.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover W. H., Martinez R. J., Rittenberg S. C. Permeability of the boundary layers of Bdellovibrio bacteriovorus 109J and its bdelloplasts to small hydrophilic molecules. J Bacteriol. 1984 Feb;157(2):385–390. doi: 10.1128/jb.157.2.385-390.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz G. W., Heppel L. A. Studies on the uptake of hexose phosphates. 3. Mechanism of uptake of glucose 1-phosphate in Escherichia coli. J Biol Chem. 1971 May 10;246(9):2891–2897. [PubMed] [Google Scholar]
- Doddema H. J., Claesen C. A., Kell D. B., van der Drift C., Vogels G. D. An adenine nucleotide translocase in the procaryote Methanobacterium thermoautotrophicum. Biochem Biophys Res Commun. 1980 Aug 14;95(3):1288–1293. doi: 10.1016/0006-291x(80)91613-7. [DOI] [PubMed] [Google Scholar]
- Eksztejn M., Varon M. Elongation and cell division in Bdellovibrio bacteriovorus. Arch Microbiol. 1977 Aug 26;114(2):175–181. doi: 10.1007/BF00410781. [DOI] [PubMed] [Google Scholar]
- Gadkari D., Stolp H. Energy metabolism of Bdellovibrio bacteriovorus. I. Energy production, ATP pool, energy charge. Arch Microbiol. 1975 Mar 10;102(3):179–185. doi: 10.1007/BF00428366. [DOI] [PubMed] [Google Scholar]
- Goulbourne E. A., Jr, Greenberg E. P. Relationship between proton motive force and motility in Spirochaeta aurantia. J Bacteriol. 1980 Sep;143(3):1450–1457. doi: 10.1128/jb.143.3.1450-1457.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P., Al-Hossainy E., Silverman J. A. Adenine nucleotide and lysine transport in Chlamydia psittaci. J Bacteriol. 1982 May;150(2):662–670. doi: 10.1128/jb.150.2.662-670.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengge R., Larson T. J., Boos W. sn-Glycerol-3-phosphate transport in Salmonella typhimurium. J Bacteriol. 1983 Jul;155(1):186–195. doi: 10.1128/jb.155.1.186-195.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hespell R. B., Mertens M. Effects of nuclei acid compounds on viability and cell composition of Bdellovibrio bacteriovorus during starvation. Arch Microbiol. 1978 Feb;116(2):151–159. doi: 10.1007/BF00406030. [DOI] [PubMed] [Google Scholar]
- Hespell R. B., Miozzari G. F., Rittenberg S. C. Ribonucleic acid destruction and synthesis during intraperiplasmic growth of Bdellovibrio bacteriovorus. J Bacteriol. 1975 Aug;123(2):481–491. doi: 10.1128/jb.123.2.481-491.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hespell R. B., Odelson D. A. Metabolism of RNA-ribose by Bdellovibrio bacteriovorus during intraperiplasmic growth on Escherichia coli. J Bacteriol. 1978 Dec;136(3):936–946. doi: 10.1128/jb.136.3.936-946.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hespell R. B., Thomashow M. F., Rittenberg S. C. Changes in cell composition and viability of Bdellovibrio bacteriovorus during starvation. Arch Microbiol. 1974 May 20;97(4):313–327. doi: 10.1007/BF00403070. [DOI] [PubMed] [Google Scholar]
- Hochman A., Bittan R., Carmeli C. Nucleotide translocation across the cytoplasmic membrane in the photosynthetic bacterium Rhodopseudomonas capsulata. FEBS Lett. 1978 May 1;89(1):21–25. doi: 10.1016/0014-5793(78)80513-4. [DOI] [PubMed] [Google Scholar]
- Kimchi A., Rosenberg E. Linkages between deoxyribonucleic acid synthesis and cell division in Myxococcus xanthus. J Bacteriol. 1976 Oct;128(1):69–79. doi: 10.1128/jb.128.1.69-79.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg M. The ADP-ATP translocation in mitochondria, a membrane potential controlled transport. J Membr Biol. 1980 Sep 30;56(2):97–105. doi: 10.1007/BF01875961. [DOI] [PubMed] [Google Scholar]
- Moulder J. W. Intracellular parasitism: life in an extreme environment. J Infect Dis. 1974 Sep;130(3):300–306. doi: 10.1093/infdis/130.3.300. [DOI] [PubMed] [Google Scholar]
- Munch-Petersen A., Mygind B. Nucleoside transport systems in Escherichia coli K12: specificity and regulation. J Cell Physiol. 1976 Dec;89(4):551–559. doi: 10.1002/jcp.1040890410. [DOI] [PubMed] [Google Scholar]
- Neale G. A., Mitchell A., Finch L. R. Uptake and utilization of deoxynucleoside 5'-monophosphates by Mycoplasma mycoides subsp. mycoides. J Bacteriol. 1984 Jun;158(3):943–947. doi: 10.1128/jb.158.3.943-947.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. Review of the Folin phenol protein quantitation method of Lowry, Rosebrough, Farr and Randall. Anal Biochem. 1979 Dec;100(2):201–220. doi: 10.1016/0003-2697(79)90222-7. [DOI] [PubMed] [Google Scholar]
- Pritchard M. A., Langley D., Rittenberg S. Effects of methotrexate on intraperiplasmic and axenic growth of Bdellovibrio bacteriovorus. J Bacteriol. 1975 Mar;121(3):1131–1136. doi: 10.1128/jb.121.3.1131-1136.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDERATH E., RANDERATH K. RESOLUTION OF COMPLEX NUCLEOTIDE MIXTURES BY TWO-DIMENSIONAL ANION-EXCHANGE THIN-LAYER CHROMATOGRAPHY. J Chromatogr. 1964 Oct;16:126–129. doi: 10.1016/s0021-9673(01)82446-8. [DOI] [PubMed] [Google Scholar]
- Rittenberg S. C., Hespell R. B. Energy efficiency of intraperiplasmic growth of Bdellovibrio bacteriovorus. J Bacteriol. 1975 Mar;121(3):1158–1165. doi: 10.1128/jb.121.3.1158-1165.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg S. C., Langley D. Utilization of nucleoside monophosphates per Se for intraperiplasmic growth of Bdellovibrio bacteriovorus. J Bacteriol. 1975 Mar;121(3):1137–1144. doi: 10.1128/jb.121.3.1137-1144.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosson R. A., Rittenberg S. C. Regulated breakdown of Escherichia coli deoxyribonucleic acid during intraperiplasmic growth of Bdellovibrio bacteriovorus 109J. J Bacteriol. 1979 Nov;140(2):620–633. doi: 10.1128/jb.140.2.620-633.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy-Burman S., Visser D. W. Transport of purines and deoxyadenosine in Escherichia coli. J Biol Chem. 1975 Dec 25;250(24):9270–9275. [PubMed] [Google Scholar]
- Ruby E. G., Rittenberg S. C. Differentiation after premature release of intraperiplasmically growing Bdellovibrio bacteriovorous. J Bacteriol. 1983 Apr;154(1):32–40. doi: 10.1128/jb.154.1.32-40.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Wentzel D. L., Feucht B. U., Judice J. J. A transport system for phosphoenolpyruvate, 2-phosphoglycerate, and 3-phosphoglycerate in Salmonella typhimurium. J Biol Chem. 1975 Jul 10;250(13):5089–5096. [PubMed] [Google Scholar]
- Seidler R. J., Starr M. P. Isolation and characterization of host-independent Bdellovibrios. J Bacteriol. 1969 Nov;100(2):769–785. doi: 10.1128/jb.100.2.769-785.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilo M., Bruff B. Lysis of Gram-negative bacteria by host-independent ectoparasitic Bdellovibrio bacteriovorus isolates. J Gen Microbiol. 1965 Sep;40(3):317–328. doi: 10.1099/00221287-40-3-317. [DOI] [PubMed] [Google Scholar]
- Simpson F. J., Robinson J. Some energy-producing systems in Bdellovibrio bacteriovorus, strain 6-5-S. Can J Biochem. 1968 Aug;46(8):865–873. doi: 10.1139/o68-129. [DOI] [PubMed] [Google Scholar]
- Thomashow M. F., Rittenberg S. C. Intraperiplasmic growth of Bdellovibrio bacteriovorus 109J: solubilization of Escherichia coli peptidoglycan. J Bacteriol. 1978 Sep;135(3):998–1007. doi: 10.1128/jb.135.3.998-1007.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. A hexose-phosphate transport system in Escherichia coli. Biochim Biophys Acta. 1966 Mar 28;117(1):231–240. doi: 10.1016/0304-4165(66)90170-x. [DOI] [PubMed] [Google Scholar]
- Winkler H. H. Rickettsial permeability. An ADP-ATP transport system. J Biol Chem. 1976 Jan 25;251(2):389–396. [PubMed] [Google Scholar]
- Yagil E., Beacham I. R. Uptake of adenosine 5'-monophosphate by Escherichia coli. J Bacteriol. 1975 Feb;121(2):401–405. doi: 10.1128/jb.121.2.401-405.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


