Figure 1.
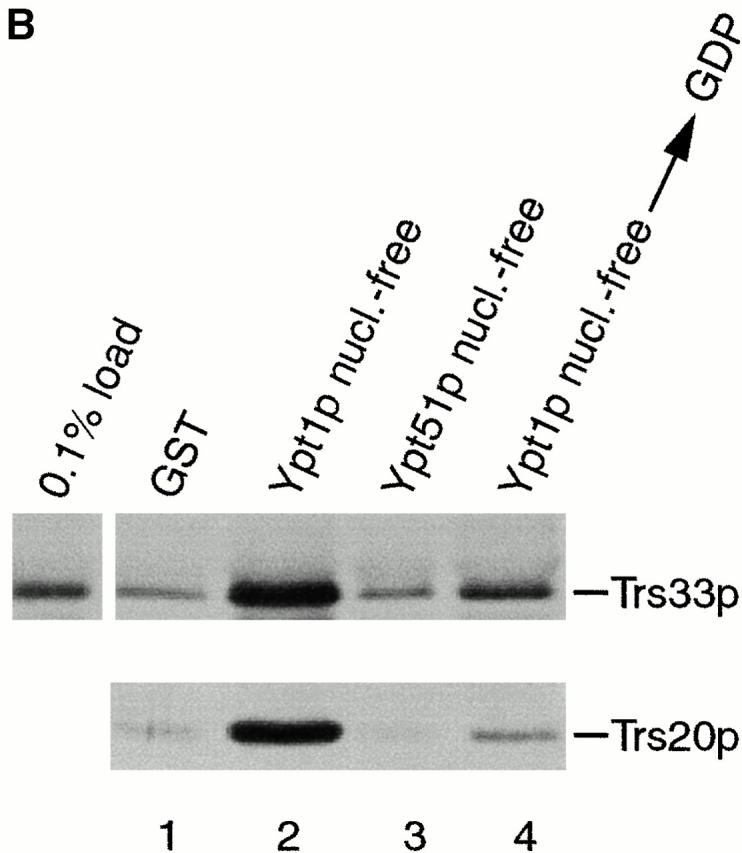
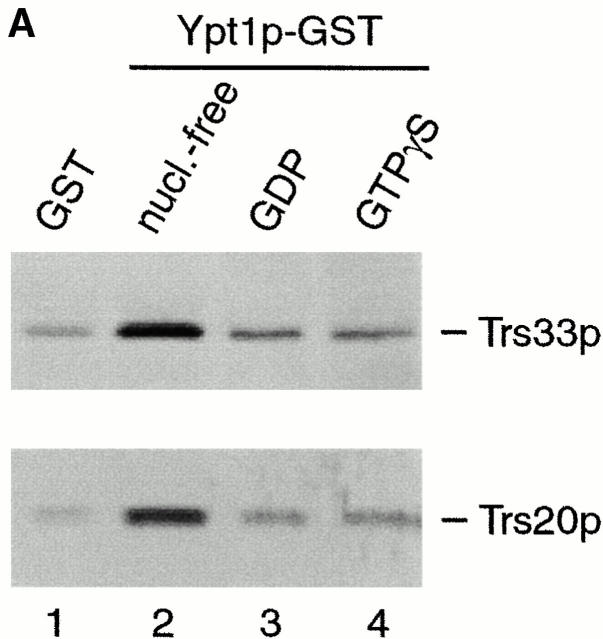
TRAPP subunits bind preferentially to the nucleotide-free form of Ypt1p. (A) A yeast lysate was incubated with agarose beads containing either GST (lane 1) or GST-Ypt1p (lanes 2–4) as described in the Materials and Methods. Before the incubation, Ypt1p was either stripped of nucleotide (nucleotide-free, lane 2) or loaded with GDP (lane 3) or GTPγS (lane 4). The beads were washed and the bound proteins were eluted by boiling in SDS-PAGE sample buffer. The eluate was then fractionated on a SDS–12.5% polyacrylamide gel, and Western blot analysis was performed by the enhanced chemiluminescence method using anti-Trs33p antibody at 1:2,500 dilution (top) or anti-Trs20p antibody at 1:1,000 dilution (bottom). (B) A yeast lysate was incubated with agarose beads containing either GST (lane 1), GST-Ypt1p (lanes 2 and 4), or GST-Ypt51p (lane 3). Before the incubation, Ypt1p and Ypt51p were stripped of nucleotide (nucleotide-free, lanes 2 and 3) or stripped of nucleotide and allowed to rebind GDP (lane 4). The beads were processed as above. The amount of Trs33p present in 0.1% of the lysate that was incubated with the beads is shown.