Figure 7.
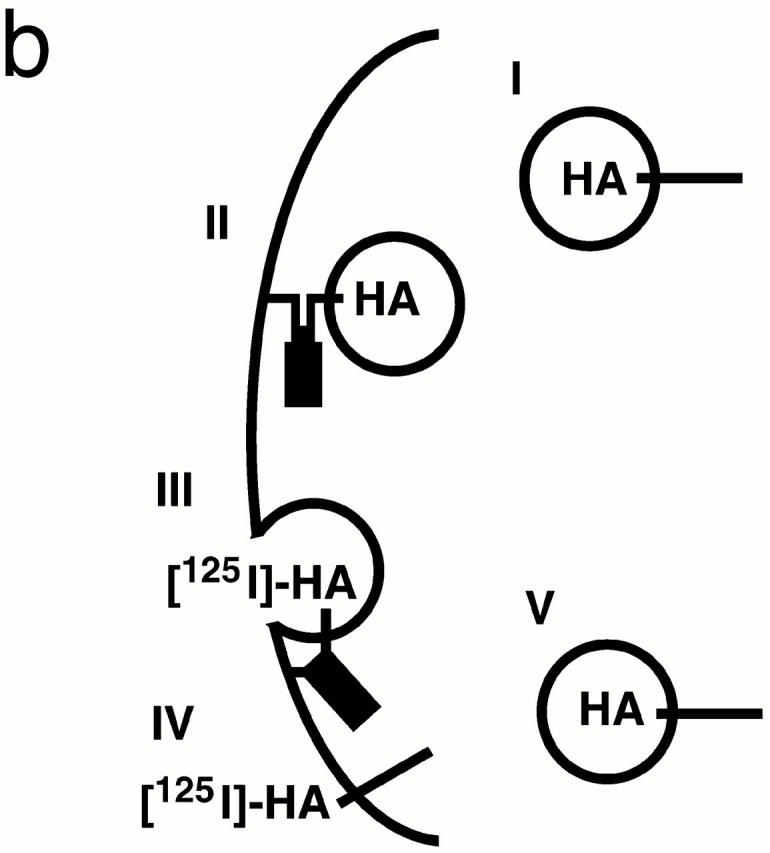
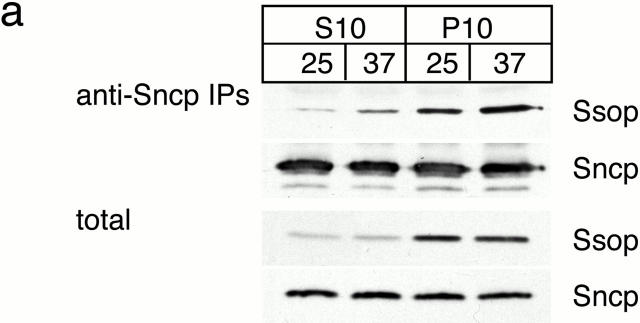
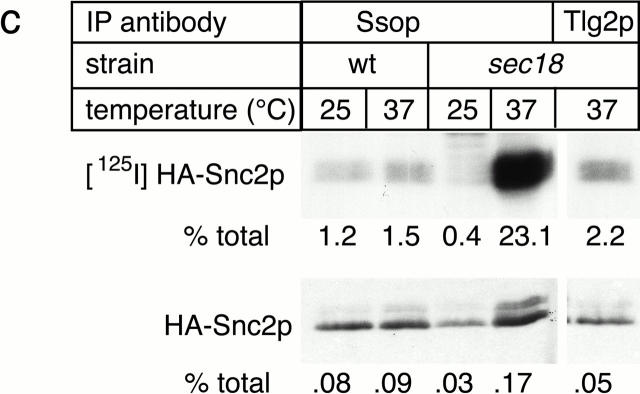
SNARE complex accumulation on the plasma membrane of sec18-1 cells. (a) Subcellular fractionation of SNAREs and SNARE complexes. sec18-1 (NY431) cells were maintained at 25°C or shifted to 37°C for 10 min, lysed in detergent-free buffer, and then fractionated into 10,000-g pellet (P) and supernatant (S) fractions. Sncp was immunoprecipitated from each fraction. An immunoblot from the total and anti-Sncp immunoprecipitated fractions was probed for Sncp and Ssop. Exposure times were varied to emphasize the distribution of Sncp and Ssop between pellet and supernatant fractions. (b) Snc2-HA in postfusion cis-SNARE complexes is accessible to surface iodination. In this model illustrating the final stages of the secretory pathway, secretory vesicles (I) dock to the plasma membrane (II) and fuse (III). After fusion, Snc2-HA on the plasma membrane (IV) recycles via endocytosis (V). Snc2-HA can be surface labeled in cis-SNARE complexes (III) but not in trans-SNARE complexes (II). (c) Coprecipitation of surface-iodinated HA-Sncp with Ssop. Aliquots of HA-Sncp expressing wild-type (NY1642) and sec18-1 (NY1643) cells were either maintained at 25°C or shifted to 37°C for 10 min. The cells were then surface labeled with 125I at 4°C, lysed, and immunoprecipitated with antibodies to Ssop and Tlg2p. Coprecipitating 125I–HA-Sncp (upper panel) was quantified by PhosphorImager analysis and compared with the total amount of 125I–HA-Sncp in an anti-HA immunoprecipitate. HA-Sncp immunoreactivity detected by Western blotting (lower panel) was quantified by densitometry and compared with the total HA-Snc2p immunoreactivity in the lysate.