Figure 4.
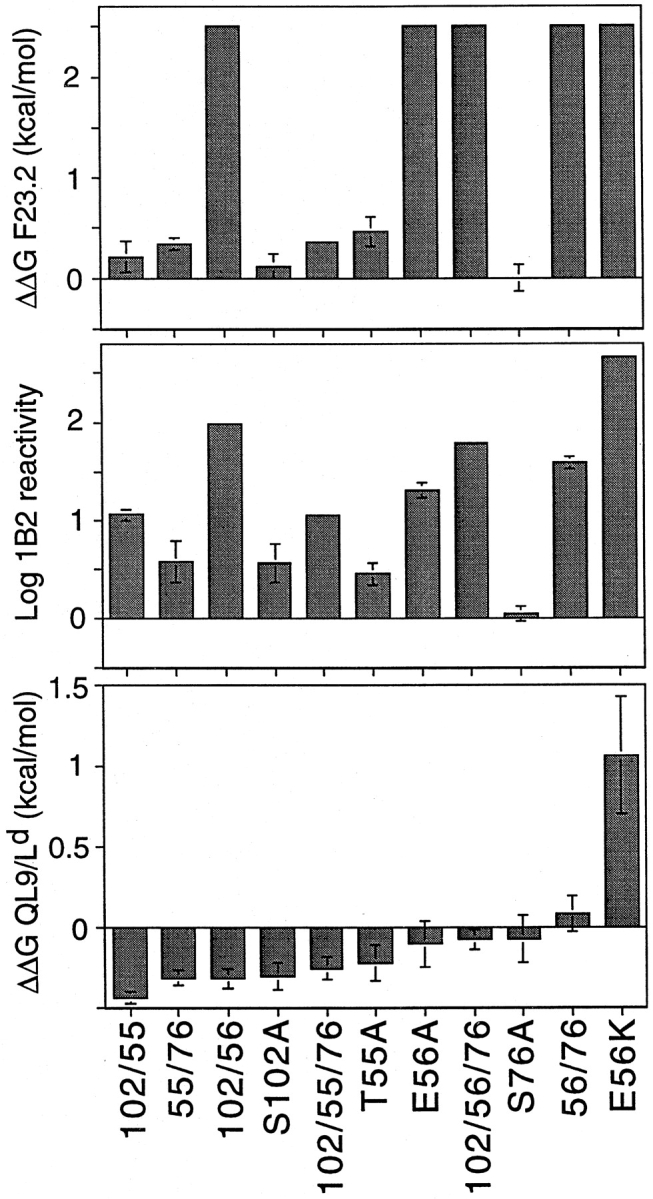
Summary binding data for alanine-substituent mutants analyzed in this study. Free energy changes for F23.2 (top) and QL9/Ld (bottom) binding were calculated from competition experiments using IC50(mutant)/IC50(wild-type) and were normalized for KJ16/F23.1 reactivity to account for slight variations in sample refolding and purity. 1B2 reactivity (middle) was determined from a capture ELISA format, with the concentration of scTCR yielding 50% maximal signal used analogously to IC50. ΔΔG values >0 indicate decreased binding.
