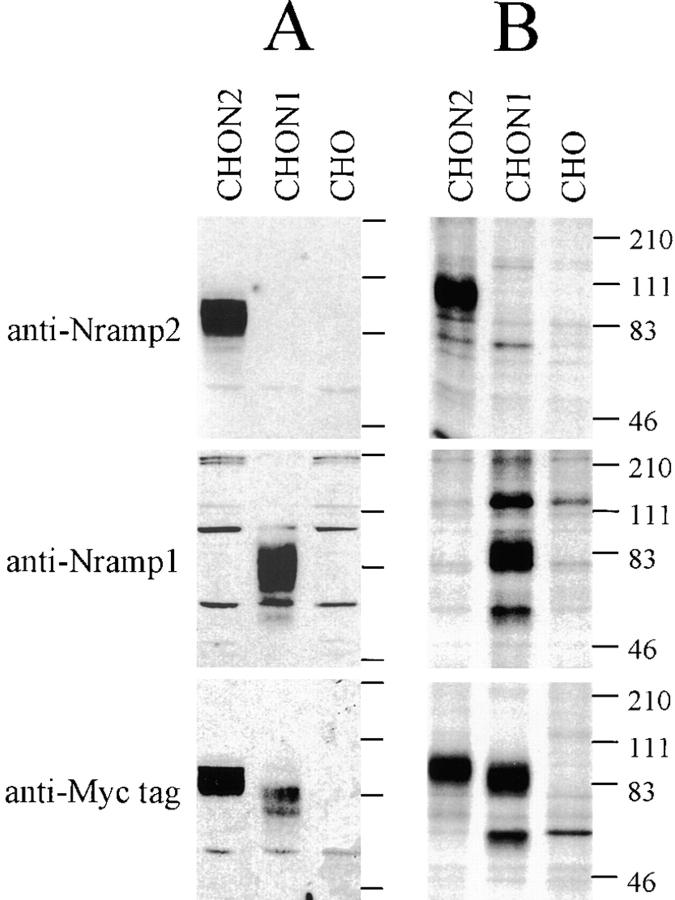
Figure 1.
After affinity purification, anti-Nramp1 and anti-Nramp2 antisera were tested for specificity and reactivity by immunoblotting against crude membrane fractions (A) as well as by immunoprecipitation of [35S]methionine–labeled cell lysates (B) from CHO cells or from the same cells transfected with a c-myc–tagged (tag sequence EQKLISEEDL) Nramp1 (CHON1) or a c-myc–tagged Nramp2 (CHON2). Protein extracts were separated by SDS-PAGE and either transferred to membranes (A) or exposed to x-ray films after immunoprecipitation (B). Antisera directed against Nramp2 (top), Nramp1 (center) or a commercially available mouse monoclonal anti–c-myc antibody (9E10; bottom) were used. The size of the molecular mass markers (in kD) is indicated to the right of the gels.