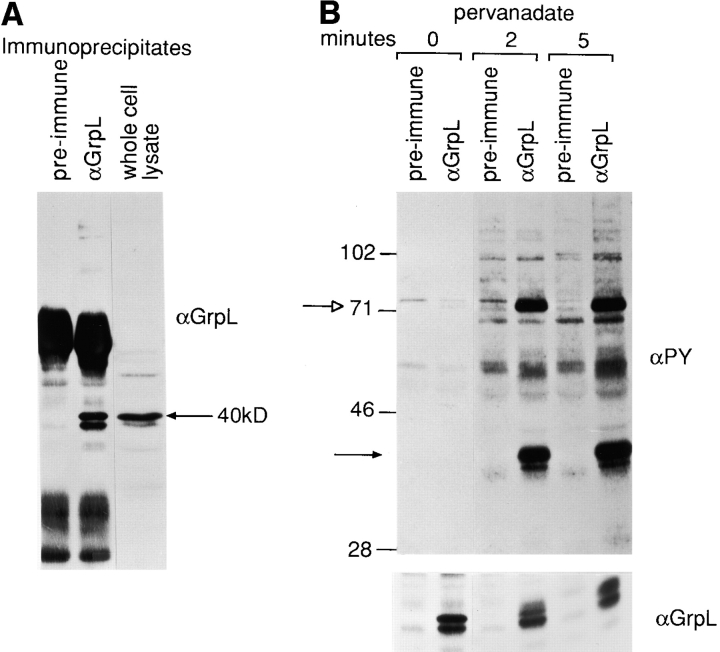
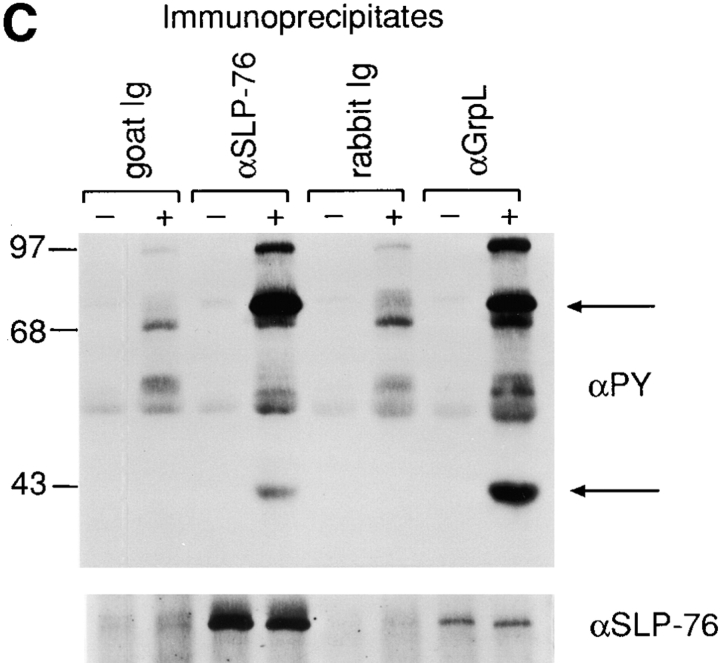
Figure 3.
Biochemical characterization of GrpL. (A) Jurkat cell lysates were immunoprecipitated with an antiserum against GrpL generated by immunizing rabbits with the GST/GrpLSH2 fusion protein or preimmune serum. IPs were resolved along with whole cell lysate on reducing SDS-PAGE and immunoblotted with the same anti-GrpL serum. (B) Jurkat cells were stimulated for either 2 or 5 min with pervanadate as described in Materials and Methods. NP-40 lysates were immunoprecipitated with anti-GrpL or preimmune serum and immunoblotted with the antiphosphotyrosine antibody 4G10 (αPY). The amounts of GrpL in different lanes were monitored by immunoblotting with anti-GrpL (αGrpL). The open arrow indicates a predominant tyrosine-phosphorylated protein coimmunoprecipitating with GrpL, and the closed arrow indicates the mobility of GrpL. (C) NP-40 lysates prepared from unstimulated (−) or pervanadate-stimulated (+) Jurkat cells were immunoprecipitated with anti–SLP-76 (αSLP-76), anti-GrpL (αGrpL), or normal goat or rabbit control Ig. IPs were resolved on SDS-PAGE and immunoblotted with either antiphosphotyrosine or anti–SLP-76. The arrows indicate phosphoproteins migrating at the sizes of SLP-76 and GrpL.