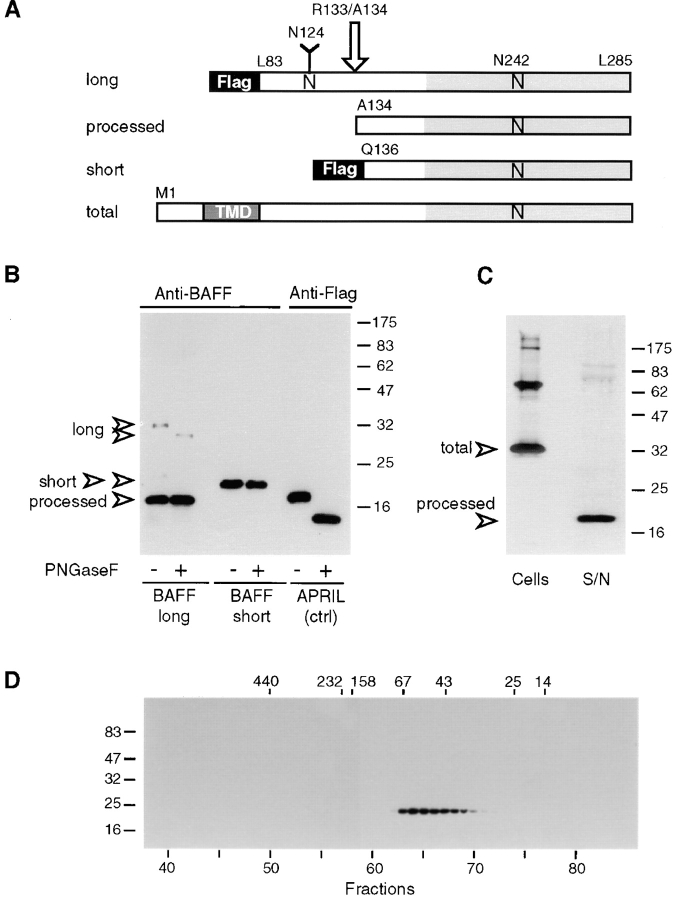
Figure 2.
Characterization of recombinant BAFF. (A) Schematic representation of recombinant BAFF constructs. Soluble recombinant BAFFs starting at Leu83 and Gln136 are expressed fused to an NH2-terminal Flag tag and a 6–amino acid linker. The long form is cleaved between Arg133 and Ala134 (arrow) in 293 T cells, to yield a processed form of BAFF. Asn124 and Asn242 belong to N-glycosylation consensus sites. N-linked glycan present on Asn124 is shown as a Y. TMD, transmembrane domain. (B) PNGase F treatment of recombinant BAFF. Concentrated supernatants containing Flag-tagged BAFFs and APRIL were deglycosylated and analyzed by Western blotting using polyclonal anti-BAFF antibodies or anti-Flag M2, as indicated. All bands except processed BAFF also reacted with anti-Flag M2 (data not shown). (C) Full-length BAFF is processed to a soluble form. 293 T cells were transiently transfected with full-length BAFF. Transfected cells and their concentrated supernatants (S/N) were analyzed by Western blotting using polyclonal anti-BAFF antibodies. Supernatants corresponding to 10 times the amount of cells were loaded onto the gel. (D) Size exclusion chromatography of sBAFF on Superdex-200. Concentrated supernatants containing sBAFF/short were fractionated on a Superdex-200 column, and the eluted fractions were analyzed by Western blotting using anti-Flag M2 antibody. The migration positions of the molecular mass markers (in kD) are indicated on the left-hand side for SDS-PAGE and at the top of the figure for size exclusion chromatography.