Abstract
We have recently described that sustained Plasmodium falciparum growth could be obtained in immunodeficient mice. We now report the potential of this new mouse model by assaying the effect of the passive transfer of antibodies (Abs) which in humans have had a well-established effect.
Our results show that the total African adult hyperimmune immunoglobulin Gs (HI-IgGs) strongly reduce P. falciparum parasitemia similarly to that reported in humans, but only when mice are concomitantly reconstituted with human monocytes (HuMNs). In contrast, neither HI-IgGs nor HuMNs alone had any direct effect upon parasitemia. We assessed the in vivo effect of epitope-specific human Abs affinity-purified on peptides derived either from the ring erythrocyte surface antigen (RESA) or the merozoite surface protein 3 (MSP3). The inoculation of low concentrations of anti-synthetic peptide from MSP3, but not of anti-RESA Abs, consistently suppressed P. falciparum in the presence of HuMNs. Parasitemia decrease was stronger and faster than that observed using HI-IgGs and as fast as that induced by chloroquine. Our observations demonstrate that this mouse model is of great value to evaluate the protective effect of different Abs with distinct specificity in the same animal, a step hardly accessible and therefore never performed before in humans.
Keywords: mouse model, protective immunity, antibody-dependent cellular inhibition, merozoite surface protein 3, ring erythrocyte surface antigen
Introduction
Malaria due to Plasmodium falciparum causes the highest mortality in individuals living in endemic countries. The development of weapons, particularly vaccines, to fight this major public health problem has been severely hampered by the lack of a suitable in vivo model. Only a very small number of primate species are receptive to P. falciparum, which scarcity and cost preclude any widespread use 1 2 3.
Animal models for falciparum malaria in immunodeficient, chimerical mice have been described recently 4 5 6. We used mice bearing the mutations beige, X-linked immunodeficient, and nude (BXN) affecting the T and B cell functions. In this model, the partial depletion of circulating and tissue phagocytes was crucial to the successful grafting of human red blood cells (HuRBCs) and to the development of the erythrocytic cycle of P. falciparum 4. After our initial report, further progress was made in controlling mouse macrophages which resulted in obtaining consistently long-lasting, stable parasitemias. In the BXN mice, the morphology of the P. falciparum parasite and the responses to currently employed antimalarial drugs were similar to those observed in humans (Moreno, A., E. Badell, N. van Rooijen, and P. Druilhe, manuscript submitted for publication), therefore confirming the validity of this in vivo P. falciparum model.
In view of the need to improve the understanding of host–parasite interactions for vaccine development, we evaluated the relevance of this P. falciparum mouse model for the analysis of the effects of different components of the human immune system on the parasite. There is considerable in vivo evidence that the IgG constitutes an efficient protective arm of the immune system against P. falciparum erythrocytic stages in humans 7 8 9. Therefore, passive transfer experiments were carried out to determine the effect of total African IgG or affinity-purified Abs against two vaccine candidates, merozoite surface protein 3 (MSP3) and ring erythrocyte surface antigen (RESA), upon the growth of P. falciparum in these mice.
Materials and Methods
Immunomodulation of Innate Immunity in BXN Mice.
6–8-wk-old male BXN mice were used. They were purchased from Charles River Laboratories, kept and manipulated under pathogen-free conditions. The protocol of immunomodulation described below represents a major improvement to our earlier protocol 4. The numbers of tissue macrophages in the peritoneum, spleen, and liver were reduced by intraperitoneal injection of 0.2 ml of dichloromethylenediphosphonate (Cl2MDP) encapsulated in liposomes 10 every 4–5 d. Cl2MDP was a gift of Roche Diagnostics (Mannheim, Germany). The increase in the number of blood polymorphonuclear neutrophils (PMNs) was controlled by intraperitoneal injections, every 3–4 d, of 300 μg of anti-PMN monoclonal Ab, NIMP-R14 11, purified from a hybridoma provided by Dr. M. Strath (National Institute for Medical Research, London, UK). We observed that mouse monocyte (MN) numbers were maintained at physiological levels in the period when these injections were made.
Grafting of Human Parasitized Red Blood Cells.
Human AB+ blood was collected on citrate phosphate dextrose (Macopharma) from donors with no previous history of malaria and centrifuged at 600 g for 10 min at 20°C. The white blood cells (buffy coat) were removed, and the packed red blood cells were suspended in sodium adenine glucose mannitol (Macopharma) and kept at 4°C for <30 d. Before use, HuRBCs were washed three times at 600 g with RPMI 1640 (GIBCO BRL). We used the P. falciparum African strains, NF54 and FCIP150, cultured in RPMI 1640 supplemented with 1 mg of hypoxanthine/liter (RPMI-Hyp) and 0.5% Albumax (GIBCO BRL). Synchronized ring forms (parasitemia between 1 and 3%) were obtained after reinvasion by flotation on plasmagel (Bellon) 12, and were washed for 5 min at 400 g in RPMI-Hyp. They were then resuspended in RPMI-Hyp at 50% hematocrit, and 2 ml of this suspension was injected intraperitoneally into each mouse on day 0. Afterwards, 2 ml of noninfected HuRBCs as described above was injected intraperitoneally at 3–4-d intervals in each mouse. The parasitemia was monitored by daily Giemsa-stained, thin blood films drawn from the tail vein. These paratized HuRBCs are hereafter referred to as P.f.-HuRBCs.
Passive Transfer Experiments in BXN Mice Grafted with P.f.-HuRBCs.
Total human peripheral blood mononuclear cells (HuPBMCs) from normal blood donors were isolated by Ficoll-hypaque 13 and washed twice with HBSS buffered with 0.01 M of Hepes (GIBCO BRL). Human monocytes (HuMNs) were actively selected by CD14+ magnetic beads using the MACS kit for magnetic cell sorting, according to the manufacturer's instructions (Miltenyi Biotec). This procedure allowed us to obtain 99% pure MN preparations with a viability >95%. The purity and viability of cellular suspension were verified by nonspecific esterase staining 14 and Trypan blue, respectively. Either 3 × 107 HuPBMCs or 3 × 106 purified HuMNs suspended in 1 ml of RPMI medium were injected intraperitoneally in each mouse. These BXN mice are hereafter referred to as P.f.-HuRBC-BXN mice.
A pool of human hyperimmune IgG was recently obtained from 200 sera collected from protected adults living in a hyperendemic area of the Ivory Coast in Africa (HI-IgG), essentially as described by Bouharoun-Tayoun et al. 15 except that the purification of IgG was made by ion-exchange chromatography on columns Q fast flow and SP sepharose fast flow (Amersham Pharmacia Biotech; reference 16). These subjects are referred to as protected since they have reached a state of clinical immunity to malaria. For passive transfer experiments, 6 mg of total HI-IgGs was injected per mouse, which corresponds to a dose of 200 mg/kg, previously shown to be effective in humans 9. Sampling of mice at regular intervals after IgG transfer and the determination of Ab concentrations by ELISA showed that mouse catabolism led to a progressive disappearance of the transferred Abs within 7 d.
To prepare anti-MSP3b and anti-RESA peptide Abs, the MSP3b with an additional COOH-terminal cysteine (MSP3b-Cys) peptide (sequence AKEASSYDYILGWEFGGGVPEHKKEEN) or the RESA peptide (sequence H-[EENVEHDA]2-[EENV]2-OH) were bound to a Sulfolink column (Pierce Chemical Co.), according to the manufacturer's instructions. 28 mg of total HI-IgGs was then loaded on these columns and ran at a rate of 1 ml/10 min at 4°C in a closed circuit for 24 h. After washing with PBS for 18 h at the same rate and temperature, a specific IgG was eluted from the column using a 0.2 M glycine solution (Sigma-Aldrich), with a pH 2.5 buffer. 1-ml fractions were collected, and the pH was immediately adjusted to 5.0 with 2 M Tris-NaOH (Sigma-Aldrich). To preserve the biological activity of the Abs, BSA was added at 2 mg/ml (Boehringer). The reactivity of each fraction against the peptide was monitored by ELISA, and those containing specific Abs were pooled and concentrated to 500 μl using a Centricon 30 (Millipore). The Abs were then dialyzed against PBS and RPMI 1640 for 24 h, sterilized on 0.220-μm Millex filters (Millipore), and stored at 4°C until use.
For passive transfer experiments, the IFA titer of the immunopurified anti-MSP3 or anti-RESA Abs was determined at the time of injection, and 250 μl/mice of these solutions was injected intraperitoneally.
Titration of Purified Abs on Native Protein.
The titer of each peptide-specific Ab preparation was determined by a 30-min incubation of serial twofold dilutions on acetone-fixed thin blood smears of P. falciparum (NF54 strain) containing either a majority of mature schizonts for anti-MSP3 Abs or a majority of ring forms for anti-RESA Abs. After washing in PBS, the slides were incubated with an anti–human, FITC-labeled secondary Ab (Biosys) diluted 1:200 in PBS supplemented with Evan's blue, 1:5,000 (Sigma-Aldrich). The titers obtained of each Ab suspension after concentration to a volume of 500 μl were: 1:200 or 1:400 for anti-MSP3 (depending on certain preparations) and 1:1,200 for anti-RESA Abs.
ELISA.
MSP3 or RESA peptide solutions at a concentration of 5 μg/ml in PBS, pH 7.4, were incubated overnight (50 μl/well) at 4°C in Maxisorb plates (Nunc). A solution of 2.5% skimmed milk in PBS was then added, and the plates were incubated for 2 h at room temperature. Aliquots of each fraction obtained in the immunopurification process of anti-MSP3 or anti-RESA Abs were diluted to 1:2 in PBS containing 1.25% skimmed milk. Afterwards, 50 μl of these dilutions was incubated in the coated plates for 1 h at room temperature, followed by an incubation of anti–human goat Fcγ fraction of IgG Abs conjugated to peroxidase (Biosys) diluted 1:2,000 in PBS containing 1.25% skimmed milk. After each incubation step, five washes were done using PBS containing 0.1% Tween 20 (Bio-Rad Laboratories). A solution containing 1 mg/ml Ortho Phenylene Diamine (Sigma-Aldrich) and l μl/ml 30% H2O2 in 0.1 M citrate buffer, pH 7.3, was used to reveal the enzymatic reaction. The optical density was measured at 450 nm using a Titertek Multiskan Mcc340 apparatus.
Results and Discussion
HI-IgGs or HuMNs Alone Have No Direct Effect In Vivo on P. falciparum.
Results obtained using the modified scheme of modulation of innate immunity in immunocompromised BXN mice are summarized in Fig. 1. In these mice, P. falciparum multiplication was obtained in a long-lasting (up to 4 mo), stable manner in HuRBCs. The range of parasitemias (0.1–5%) was lower than in South American primates 2 17 18 but closer to that usually observed in humans 19 20, with moderate daily variations similar to those seen in untreated patients 20 21. The level and consistency of the parasitemia in our model were thus considered suitable to envisage studies of the role of immune effectors.
Figure 1.
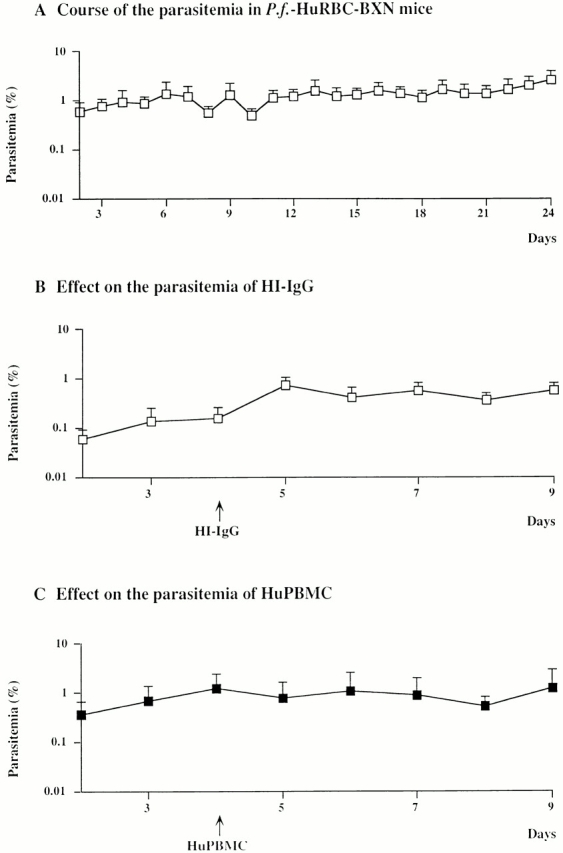
In vivo transfer of either HI-IgGs or HuPBMCs in P.f.-HuRBC-BXN mice. Course of P. falciparum parasitemia in P.f.-HuRBC-BXN mice undergoing the complementary immunomodulation protocol, injected intraperitoneally on day 0 with P. falciparum–infected HuRBCs. Noninfected HuRBCs were injected subsequently at 3–4-d intervals. (A) Shown is the mean ± SD from the parasitemia in seven representative BXN mice. (B) Mean parasitemia in three P.f.-HuRBC-BXN mice after intraperitoneal injection of HI-IgGs at a dose of 200 mg/kg. (C) Mean parasitemia in five P.f.-HuRBC-BXN mice after intraperitoneal injection of 30 × 106 HuPBMCs/mouse. Arrows, day of injection.
The injection of HI-IgGs, in similar quantities (200 mg/kg) to those used in passive transfer experiments in humans 9, surprisingly induced no significant change in the course of parasitemias in the P.f.-HuRBC-BXN mice (Fig. 1 B). These results were consistent among five similarly treated mice, despite the fact that substantial levels of human Abs could indeed be detected by ELISA (up to 8 d in all) in the injected mice (data not shown).
These observations differed from those made after the passive transfer in immunocompetent humans 9 but were reminiscent of those obtained under in vitro conditions 15 22. We had previously shown that HI-IgGs alone had no effect on P. falciparum in vitro, but a potent inhibitory effect is obtained when IgGs cooperate with blood MNs. This Ab-dependent, cell-mediated cytotoxicity-like cooperation was termed Ab-dependent cellular inhibition (ADCI [15, 23, 24]). The current observation suggested that in our model, MNs from treated mice were unable to cooperate with human IgGs in an ADCI fashion. This raised the possibility that MNs of human origin are required. We first investigated the effect of HuMNs alone, since it has been reported that MNs can nonspecifically inhibit parasite growth 25. We injected either total HuPBMCs (3 × 107) or purified HuMNs (3 × 106) into infected mice. The injection of 3 × 107 HuPBMCs resulted in the detection by FACS® of 800 HuMNs/μl of mouse blood (data not shown), i.e., close to physiological levels. As shown in Fig. 1 C and Fig. 2B and Fig. C, the injection of either HuPBMCs or purified MNs had no direct effect on the course of parasitemia at the concentrations employed. These results were consistently obtained in a total of eight different mice.
Figure 2.
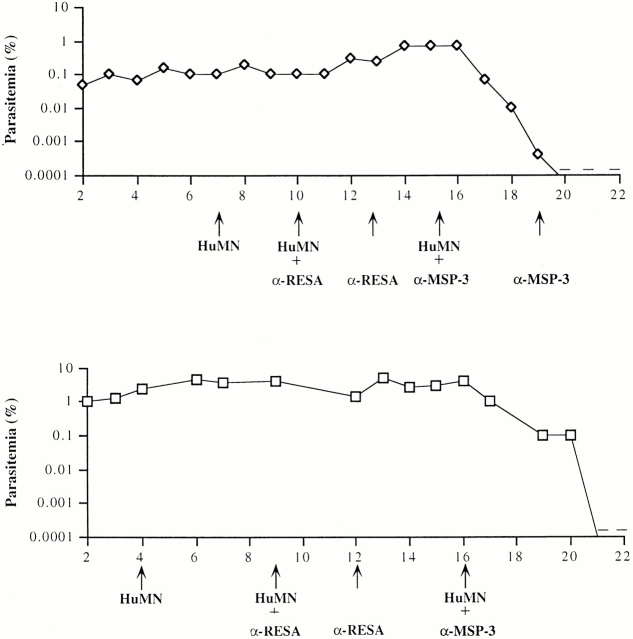
In vivo transfer of HI-IgGs together with HuPBMCs (A and B) or MNs (C and D) in P.f.-HuRBC-BXN mice. Individual parasitemia in two out of four mice injected sequentially with either 200 mg/kg of HI-IgGs at day 9, followed 6 d later by HuPBMCs (3 × 107) (A) or the same in the reverse order on days 6 and 9, respectively (B). Arrows show treatment days. In mouse A (but not in mouse B) an additional dose of 100 mg/kg of HI-IgGs was added because of the longer delay between the two treatments and the fast catabolism of human IgGs. Two other mice undergoing the same protocol showed the same effect on the course of P. falciparum parasitemia. C and D show the individual parasitemia of two out of four mice undergoing the same protocol except that purified HuMNs were used at a rate of 3 × 106 ntraperitoneally instead of total PBMCs, together with 200 mg/kg of HI-IgGs. In mouse D, only the initial IgG injection was of 200mg/kg and the further two were of 100 mg/kg. In two other mice, not shown in the figure, receiving IgG and/or MNs on days 10 and 15, or 16 and 22, respectively, the decrease of parasitemia occurred over 4 d after the first combined injection. Only mouse D showed a partial response to treatment. +, one or two parasites observed in thin blood smears; −, parasites no longer detectable in thin blood smears.
HI-IgGs and HuMNs Are Needed to Control P. falciparum In Vivo in Mice.
The medium-grade stable parasitemias that can be obtained in immunocompromised mice allow one to perform sequential studies with several immune effectors. One infected mouse was first injected with HI-IgGs alone and then received HuPBMCs plus HI-IgGs 6 d later (Fig. 2 A). In another infected mouse, HuPBMCs were first injected and Hu-IgG was only added 3 d later (Fig. 2 B). When initially added alone, the two immune components showed no inhibitory effect on the parasitemia, but when present together a strong reduction of parasitemia was observed. Duplicate results were obtained in two other mice treated in the same fashion (data not shown). The role of MNs in parasite growth inhibition was demonstrated by injecting enriched human MNs, instead of HuPBMCs, together with HI-IgGs. A similar inhibitory effect on parasite growth was observed (Fig. 2C and Fig. D) in these and duplicate experiments, irrespective of the order of injection. The reduction in parasitemia observed in mice, although strong, was nonetheless subcurative, i.e., a low grade parasitemia persisted for several days. This was also the case in passive transfers in humans 9.
Immunopurified Anti-MSP3, but Not Anti-RESA Abs, Inhibit Parasite Growth.
The results obtained using total HI-IgGs and HuMNs established the model potential for assaying the effect of Abs and led us to evaluate the protective effect of Abs with defined specificity, a step hardly accessible and therefore never performed before in humans. To this end, we affinity-purified Abs from the HI-IgG pool on epitopic peptides. We chose MSP3b and the RESA peptide, derived from the two vaccine candidates MSP3 and RESA, respectively. Before the passive transfers, the specificity and titers of the purified Abs were assessed against both the original peptides and the native parasite antigens. Both sets of Abs reacted specifically at 1:400 dilution for the anti-MSP3 Abs and 1:1,200 for the anti-RESA Abs (Table ).
Table 1.
Specificity of Immunopurified Abs against MSP3 and RESA Peptides Detected by IFA and ELISA
| IFA | ELISA | |||
|---|---|---|---|---|
| Abs | Schizonts | Rings | MSP3 | RESA |
| N-IgG | (−) | (−) | 58 | 62 |
| HI-IgG | 1/12,000 | 1/12,000 | 908 (16) | 859 (14) |
| MSP3b-IgG | 1/400 | (−) | 942 (16) | 39 (0.6) |
| RESA-IgG | (−) | 1/1,200 | 37 (0.6) | 821 (13) |
IFA was performed on slides containing a high percentage of ring forms for anti–RESA-IgG and a high percentage of schizonts for anti–MSP3b-IgG. The ratio (in parentheses) was calculated by dividing the optical density of each IgG preparation by the optical density of normal human IgG (N-IgG) against each peptide.
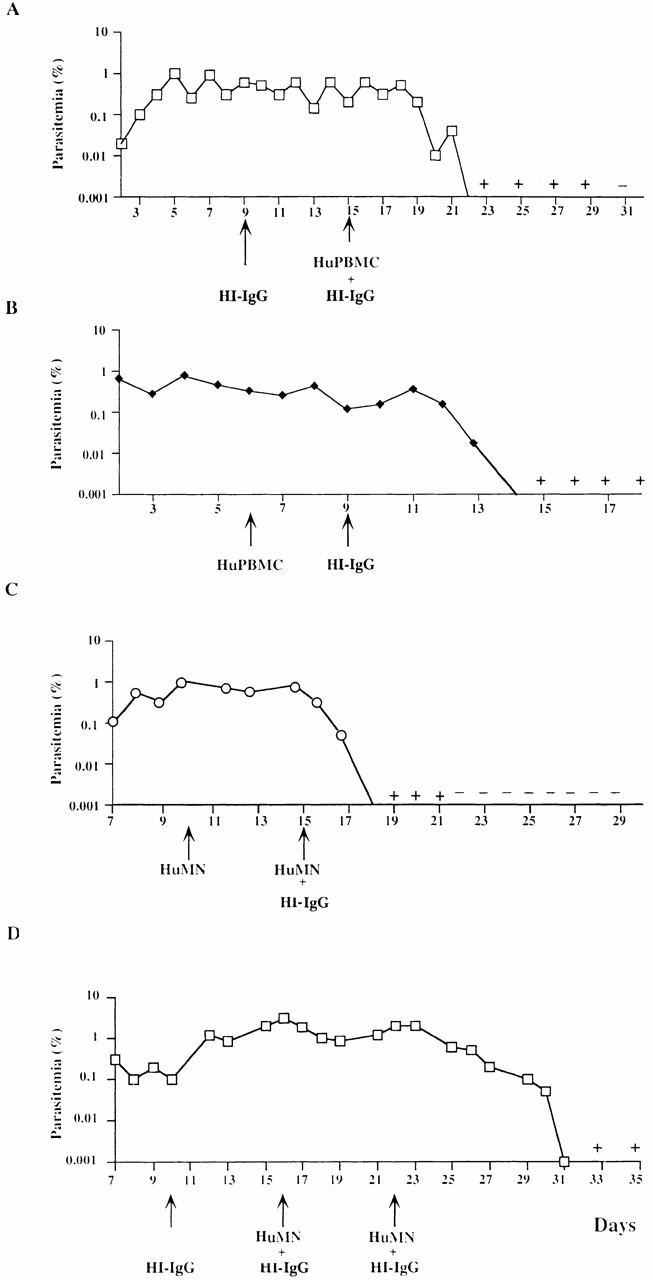
The in vivo effect of the two purified Abs was investigated using the sequential procedure described above, where each mouse served as its own control. The transfer of anti-MSP3 Ab or purified HuMNs alone had no effect on P. falciparum parasitemia (Fig. 3A and Fig. B). However, the addition of HuMNs followed 3 d later by the injection of the specific Ab induced a fast, strong, and consistent decrease in parasitemia (Fig. 3C and Fig. D). On average, the decrease (>10-fold/48 h) was stronger and faster than that observed with total HI-IgG (Fig. 2 C), and as fast as that observed when using chloroquine either in P.f.-HuRBC-BXN mice (Moreno, A., E. Badell, N. van Rooijen, and P. Druilhe, manuscript submitted for publication) or in humans 26. These results were reproduced in six different mice, in three distinct experiments using three separate batches of Abs and HuMNs. By contrast, the parasitemia in two other mice remained unchanged when a similar course of injections was performed with anti-RESA Abs and HuMNs (Fig. 4A and Fig. B). However, parasite numbers dropped drastically when the same mice were further injected with anti-MSP3 Abs and HuMNs 3–4 d later. Finally, and in contrast to results obtained with HI-IgG, five out of six mice completely cleared the parasitemia.
Figure 3.
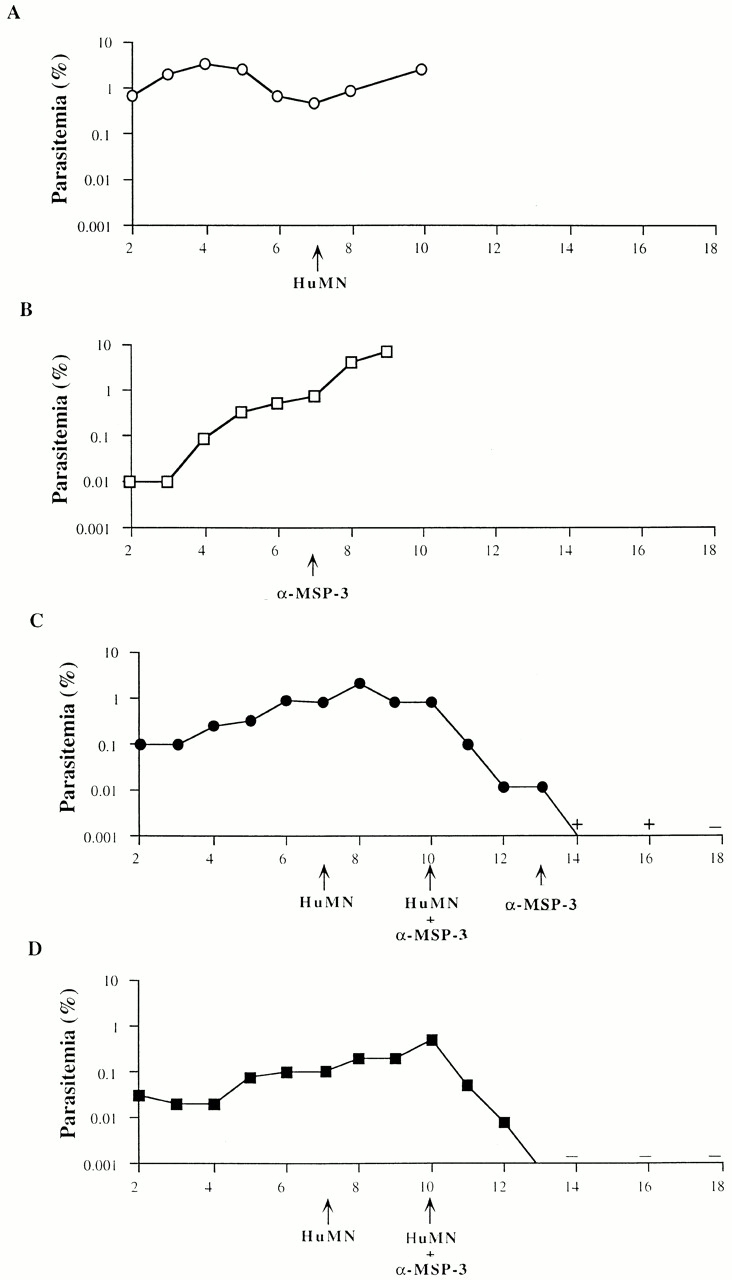
In vivo transfer of anti-MSP3b peptide Abs with HuMNs in P.f.-HuRBC-BXN mice. (A) Course of parasitemia in a mouse receiving a single injection of HuMNs (3 × 106). (B) Parasitemia in a mouse receiving a single injection of anti-MSP3b peptide–specific human Abs (at a rate of 250 μl of a solution titrating at 1:200 by IFA). (C and D) Course of parasitemia in two mice receiving first HuMNs (3 × 106) and thereafter the anti-MSP3b Abs (250 μl) together with an additional dose of HuMNs (3 × 106). + and −, presence or absence of few residual parasites in thin blood smears. Arrows, day of injection.
Figure 4.
Sequential effect of anti-RESA and anti-MSP3b Abs. Course of parasitemia in two P.f.-HuRBC-BXN mice receiving sequential treatment first by HuMNs, then by HuMNs together with anti-RESA peptide Abs (250 μl of a solution with a titer of 1:2,000 by IFA), and followed by HuMNs together with anti-MSP3b peptide Abs (250 μl, IFA 1:200). The arrows show the days at which treatment was administered. −, absence of residual parasites in thin blood smears.
It is striking that such a profound, biological effect was obtained using a very minor subset of all antimalarial Abs present in the hyperimmune serum. Nonetheless, there is convergence with data obtained by other approaches. The strong effect of low amounts of anti-MSP3 Abs in BXN mice is in keeping with indications previously gathered under in vitro conditions 27; also, recently obtained immunoepidemiological data further corroborate the protective role of low amounts of anti-MSP3b peptide IgG3 Abs (Roussilhon, C., C. Oeuvray, J.L. Perignon, and P. Druilhe, manuscript in preparation). The lack of inhibition obtained with anti-RESA Abs clearly does not rule out a role for this molecule, particularly since these were purified on a short peptide.
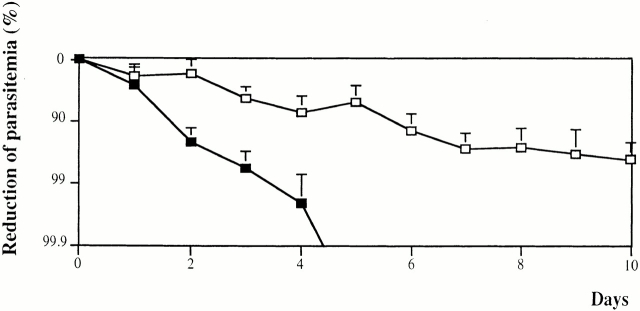
Fig. 5 shows the rate of parasitemia decrease in two groups of six mice treated either by total African HI-IgGs or affinity-purified anti-MSP3 Abs, with both cases receiving human MNs. Anti-MSP3b peptide Abs achieved a fast and total clearance of parasites, whereas HI-IgG induced a slower rate of parasite clearance (P < 0.05), leaving a chronic, low-grade parasitemia. It should be noted that the titer of affinity-purified, anti-MSP3b Abs was adjusted to be the same as that in total HI-IgGs (Table ), i.e., total HI-IgGs contained the same amounts of anti-MSP3b Abs that were present in the purified preparation. This observation indirectly implies that other Abs present in HI-IgG might block the inhibitory effect of anti-MSP3 Abs. Such a blocking effect is reminiscent of that observed with anti-MSP1 Abs 28, or could also be related to noncytophilic Abs directed to other merozoite surface antigens, resulting in steric hindrance. This observation has important implications for vaccine development, as it can be taken as an indication that immunization by selected malarial antigens may elicit stronger protective responses than those resulting from exposure to all malarial proteins. In other words, the immunization by molecules which are proven targets of protective mechanisms may lead to induce a stronger protection than that developed by natural exposure.
Figure 5.
Decrease of parasitemia in HI-IgG– and anti-MSP3b–treated mice. Comparison between the mean decrease in parasitemias (± SD) in two groups of six mice, one treated by total African HI-IgGs (□), the other by affinity-purified anti-MSP3 Abs (▪). In both groups, HuMNs and Abs were injected on day 0. Note that the percentage of reduction of parasitemia is shown as a logarithmic scale.
For this type of study, the in vivo BXN model offers several significant advantages over in vitro assays: (a) in mice, the liver, kidney, and pulmonary functions provide a constant physiological environment with optimally regulated pH, electrolyte balance, glucose regulation, and nutrient supply which are known to vary in vitro, and toxic substances produced by the parasite (e.g., lactate, pigment) are efficiently scavenged by the mouse; (b) serum lipids preserve the integrity of human cell membranes whereas erythrocytes are fragilized in vitro 29; (c) in vitro MN adherence to plastic triggers their fast differentiation into macrophages which are inefficient in ADCI 30, which was not seen in mice (data not shown); and (d) in static cultures, inhibitory effectors produced by HuMNs 24 are in direct contact with P. falciparum. The fact that parasite growth inhibition is seen in the mice, despite the dilution of these monokines by the circulation, indicates that they are indeed highly potent.
In conclusion, the novel P. falciparum mouse model can now be employed for rapid in vivo investigations of the protective role of antigen-specific Abs. It allows one to employ very small amounts of defined Abs to investigate the protective role of a given antigen expressed by malarial parasites. In this respect, it stands aside from current strategies relying on the immunization of rare and costly primates susceptible to P. falciparum. Moreover, the two approaches differ also in their concept: Abs induced by artificial immunization may differ in their protective effect from naturally acquired Abs due to differences in their fine epitope specificity or isotype profile. Given the very large number of malarial molecules which are potential vaccine candidates, the P. falciparum mouse model could provide an efficient means to select those most suitable to initiate vaccine development.
Acknowledgments
We thank Georges Snounou for his essential contribution, and Daniel Scott, Jean L. Pérignon, and members of the Bio-Medical Parasitology Unit for their critical reading of the manuscript and helpful comments.
This work was supported by European Community grant 940317, and by World Health Organization grant ID 960 617.
References
- Young M.D., Baerg D.C., Rossan R.N. Parasitological review. Experimental monkey hosts for human plasmodia. Exp. Parasitol. 1975;38:136–152. doi: 10.1016/0014-4894(75)90046-6. [DOI] [PubMed] [Google Scholar]
- Taylor D.W., Siddiqui W.A. Susceptibility of owl monkeys to Plasmodium falciparum infection in relation to location of origin, phenotype, and karyotype. J. Parasitol. 1979;65:267–271. [PubMed] [Google Scholar]
- Gysin J., Fandeur T. Saimiri sciureus (karyotype 14-7)an alternative experimental model of Plasmodium falciparum infection. Am. J. Trop. Med. Hyg. 1983;32:461–467. doi: 10.4269/ajtmh.1983.32.461. [DOI] [PubMed] [Google Scholar]
- Badell E., Pasquetto V., Van Rooijen N., Druilhe P. A mouse model for human malaria erythrocytic stages. Parasitol. Today. 1995;11:235–237. doi: 10.1016/0169-4758(95)80147-2. [DOI] [PubMed] [Google Scholar]
- Moore J.M., Kumar N., Shultz L.D., Rajan T.V. Maintenance of the human malarial parasite, Plasmodium falciparum, in scid mice and transmission of gametocytes to mosquitoes. J. Exp. Med. 1995;181:2265–2270. doi: 10.1084/jem.181.6.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji M., Ishihara C., Arai S., Hiratai R., Azuma I. Establishment of a SCID mouse model having circulating human red blood cells and a possible growth of Plasmodium falciparum in the mouse. Vaccine. 1995;13:1389–1392. doi: 10.1016/0264-410x(95)00081-b. [DOI] [PubMed] [Google Scholar]
- Cohen S., McGregor A., Carrington S. Gamma globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- Edozien J.C., Gilles H.M., Udeozo I.O.K. Adult and cord-blood immunoglobulin immunity to malaria in Nigerians. Lancet. 1962;2:951–955. [Google Scholar]
- Sabchareon A., Burnouf T., Ouattara D., Attanath P., Bouharoun-Tayoun H., Chantavanich P., Foucault C., Chongsuphajaisiddhi T., Druilhe P. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am. J. Trop. Med. Hyg. 1991;45:297–308. doi: 10.4269/ajtmh.1991.45.297. [DOI] [PubMed] [Google Scholar]
- Van Rooijen N., Sanders A. Liposome mediated depletion of macrophagesmechanism of action, preparation of liposomes and applications. J. Immunol. Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Lopez A.F., Strath M., Sanderson C.J. Differentiation antigens on mouse eosinophils and neutrophils identified by monoclonal antibodies. Br. J. Haematol. 1984;57:489–494. doi: 10.1111/j.1365-2141.1984.tb02923.x. [DOI] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J.P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Investig. Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Tucker S.B., Pierre R.V., Jordon R.E. Rapid identification of monocytes in a mixed mononuclear cell preparation. J. Immunol. Methods. 1977;14:267–269. doi: 10.1016/0022-1759(77)90137-5. [DOI] [PubMed] [Google Scholar]
- Bouharoun-Tayoun H., Attanath P., Sabchareon A., Chongsuphajaisiddhi T., Druilhe P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J. Exp. Med. 1990;172:1633–1641. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blancher A., Socha W.W., Roubinet F., Rowe A.W., Broly H., Byrne P., Holuigue M., Bouzidi A., Huart J.J., Ruffie J. Human monoclonal anti-D-induced clearance of human D-positive red cells in a chimpanzee model. Vox Sang. 1993;65:47–54. doi: 10.1111/j.1423-0410.1993.tb04524.x. [DOI] [PubMed] [Google Scholar]
- Wellde B.T., Johnson A.J., Williams J.S., Sadun E.H. Experimental infection with Plasmodium falciparum in Aotus monkeys. I. Parasitologic, hematologic, and serum biochemical determinations. Am. J. Trop. Med. Hyg. 1972;21:260–271. doi: 10.4269/ajtmh.1972.21.260. [DOI] [PubMed] [Google Scholar]
- Collins W.E., Stanfill P.S., Skinner J.C., Harrison A.J., Smith C.S. Studies on human malaria in Aotus monkeys. IV. Development of Plasmodium falciparum in two subspecies of Aotus trivirgatus . J. Parasitol. 1974;60:355–358. [PubMed] [Google Scholar]
- Kitchen S.F. Symptomatologygeneral considerations. In: Boyd M.F., editor. Malariology. W.B. Saunders Co; Philadelphia/London: 1949. pp. 966–1045. [Google Scholar]
- Gravenor M.B., McLean A.R., Kwiatkowski D. The regulation of malaria parasitaemiaparameter estimates for a population model. Parasitology. 1995;110:115–122. doi: 10.1017/s0031182000063861. [DOI] [PubMed] [Google Scholar]
- Farnert A., Snounou G., Rooth I., Bjorkman A. Daily dynamics of Plasmodium falciparum subpopulations in asymptomatic children in a holoendemic area. Am. J. Trop. Med. Hyg. 1997;56:538–547. doi: 10.4269/ajtmh.1997.56.538. [DOI] [PubMed] [Google Scholar]
- Khusmith S., Druilhe P. Cooperation between antibodies and monocytes that inhibit in vitro proliferation of Plasmodium falciparum . Infect. Immun. 1983;41:219–223. doi: 10.1128/iai.41.1.219-223.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouharoun-Tayoun H., Druilhe P. Plasmodium falciparum malariaevidence for an isotype imbalance which may be responsible for delayed acquisition of protective immunity. Infect. Immun. 1992;60:1473–1481. doi: 10.1128/iai.60.4.1473-1481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouharoun-Tayoun H., Oeuvray C., Lunel F., Druilhe P. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J. Exp. Med. 1995;182:409–418. doi: 10.1084/jem.182.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan K., Dubey M.L., Ganguly N.K., Mahajan R.C. Plasmodium falciparumrole of activated blood monocytes in erythrocyte membrane damage and red cell loss during malaria. Exp. Parasitol. 1995;80:54–63. doi: 10.1006/expr.1995.1007. [DOI] [PubMed] [Google Scholar]
- Fowler V.G., Jr., Lemnge M., Irare S.G., Malecela E., Mhina J., Mtui S., Mashaka M., Mtoi R. Efficacy of chloroquine on Plasmodium falciparum transmitted at Amani, eastern Usambara Mountains, north-east Tanzaniaan area where malaria has recently become endemic. J. Trop. Med. Hyg. 1993;96:337–345. [PubMed] [Google Scholar]
- Oeuvray C., Bouharoun-Tayoun H., Gras-Masse H., Bottius E., Kaidoh T., Aikawa M., Filgueira M.C., Tartar A., Druilhe P. Merozoite surface protein-3a malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. Blood. 1994;84:1594–1602. [PubMed] [Google Scholar]
- Guevara Patino J.A., Holder A.A., McBride J.S., Blackman M.J. Antibodies that inhibit malaria merozoite surface protein-1 processing and erythrocyte invasion are blocked by naturally acquired human antibodies. J. Exp. Med. 1997;186:1689–1699. doi: 10.1084/jem.186.10.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dei-Cas E., Wattez A., Vernes A. Influence of medium osmolality on the in vitro growth of Plasmodium falciparuma morphologic and radioisotopic study. In Vitro Cell. Dev. Biol. 1985;21:93–98. doi: 10.1007/BF02620949. [DOI] [PubMed] [Google Scholar]
- Lunel F., Druilhe P. Effector cells involved in nonspecific and antibody-dependent mechanisms directed against Plasmodium falciparum blood stages in vitro. Infect. Immun. 1989;57:2043–2049. doi: 10.1128/iai.57.7.2043-2049.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]