Figure 1.
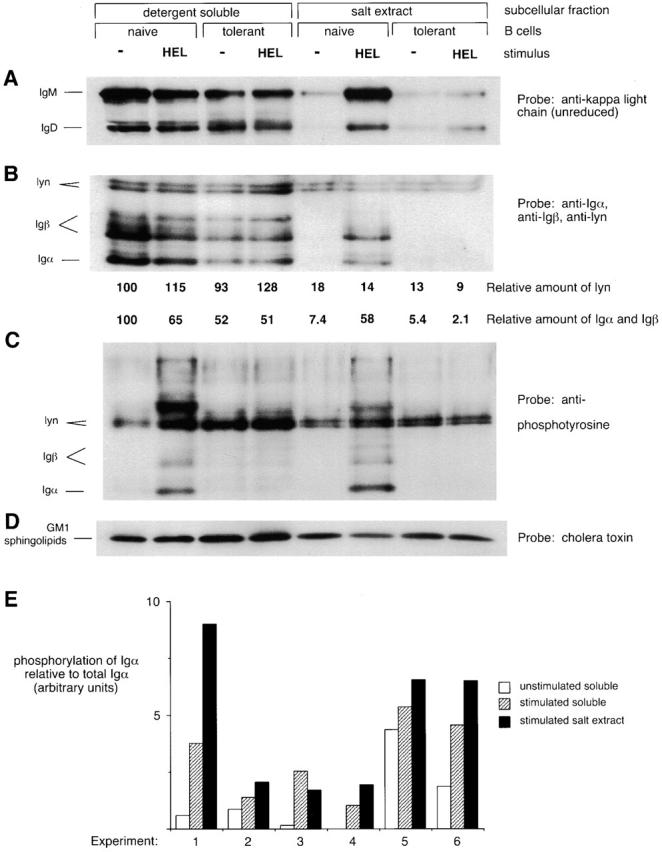
BCRs accumulate in a subcellular compartment that is detergent insoluble and salt extractable after antigen stimulation of naive but not tolerant B cells. Spleen cells from anti-HEL BCR transgenic (naive B cells) or anti-HEL/soluble HEL double-transgenic mice (tolerant B cells) were mock stimulated or stimulated with 10 μg/ml HEL for 3 min and then fractionated to obtain the detergent-soluble supernatant and the detergent-insoluble salt extract. The salt extract lanes represent three times as many cell equivalents as the detergent-soluble lanes. (A) Unreduced SDS-PAGE Western blots were probed with anti–κ L chain to resolve IgM and IgD H chain–L chain BCR complexes. (B–D) Reduced SDS-PAGE Western blots were probed (B) with a mixture of anti-Igα, anti-Igβ, and anti-lyn; (C) with 4G10 antiphosphotyrosine; (D) with cholera toxin, which binds to GM1 sphingolipids running with the dye front. In B, numbers below each lane are the amount of lyn immunoreactivity, corrected for loading, and the amount of Igα and Igβ immunoreactivity normalized to the average amount of lyn in each fraction. Quantitation was determined by PhosphorImager analysis of 125I–Protein A-developed blots. (E) Data from six independent experiments show that Igα in the salt extract fraction is more highly phosphorylated than Igα in the soluble fraction after stimulation of naive B cells. The degree of phosphorylation of Igα was obtained by dividing the intensity of the Igα-sized band on the antiphosphotyrosine blot by the intensity of the Igα band on the same blot reprobed with anti-Igα. The degree of Igα phosphorylation induced in the extract was significantly different from that induced in the supernatant to P < 0.1.
