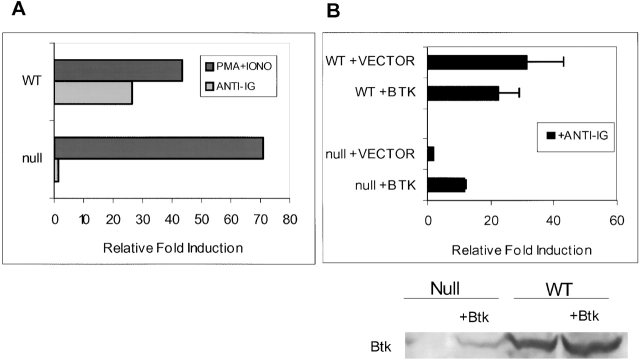
Figure 4.
Reconstitution of IgM-mediated NF-κB activity in BTK-deficient DT40 cells by ectopic expression of wild-type (WT) BTK. (A) Transient transfections of WT and BTK-deficient DT40 cells were conducted with NF-κB–driven luciferase reporter and pRLTK constructs, the latter serving as an internal transfection control for the assay. Cells were cultured for 16 h and then stimulated for 4 h with medium, M4 anti-chicken IgM (10 μg/m; light bars), or PMA and ionomycin (30 nM and 1 mM, respectively; dark bars). Cells were lysed and assayed as described in Materials and Methods. For each sample, luciferase activity was normalized to the pRLTK internal control. The graph shows the fold induction of luciferase activity relative to the luciferase activity detected in the lysates of cells cultured in medium only (= 1). This figure is representative of five experiments. (B) 10 μg of wild-type BTK or vector (pApuro) was transiently transfected together with reporter and internal control constructs as described above (A). Null + Vector lane shows background activity levels. PMA and ionomycin treatment of these cells resulted in similar induction (data not shown). Western blot analysis for detection of BTK expression was performed using lysates (equivalent of 2.5 × 106 cells) from this assay. The anti-BTK antibody recognizes both mouse and chicken BTK protein. The reactivity of this antibody for the different BTK species is not known, and the fraction of cells transfected was not determined the expression levels in wild-type and transfected cells cannot be compared. The fold induction values are the mean of three experiments ± SD.