Figure 2.
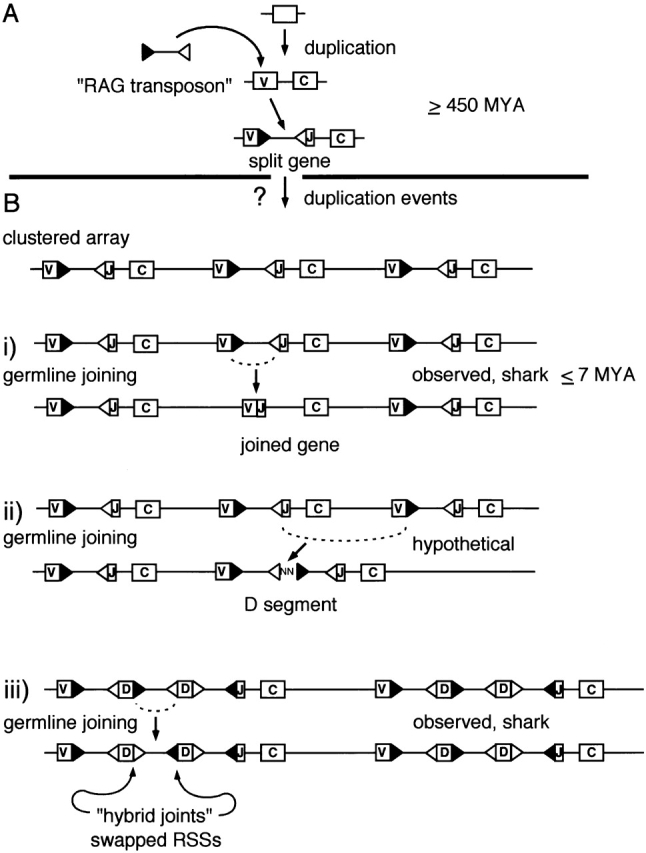
Postulated intermediates in the molecular evolution of the Ig and TCR loci. (A) One theory is that the event responsible for the existence of the modular recombination units characterizing today's Ig and TCR loci was a singularity that took place early in vertebrate evolution. With the integration of a mobile element, an ancient gene encoding an Ig superfamily domain was split into two parts. The mobile element imported RAG recombinase, its RSS, and possibly other element-related features, into the vertebrate genome. Reassembly of a functional exon required site-specific excision of the introduced mobile element, through a RAG-mediated recombination event targeting the RSS motifs. (B) After the RAG transposon integrated, the interrupted gene was duplicated. It has been suggested by Litman et al. (reference 2) that the presence of clustered arrays of duplicated genes, as found today in sharks, resembles an early locus configuration. On this view, V(D)J recombination activity in germline tissues could have led to the following derived features: (i) joined copies, as seen in cartilaginous fish, arose through a “standard” V(D)J joining event resulting in coding joint formation; (ii) the de novo creation of D segments could have arisen from intercluster recombinations resulting in the formation of signal joints with junctional insertions; (iii) the substitution of a 12-spacer RSS for a 23-spacer RSS or vice versa may have resulted from the “hybrid joint” outcome of germline joining. All of the postulated manipulations are RAG mediated and site specific. Events shown in i and iii are supported by evidence from sharks. MYA, million years ago.
