Figure 4.
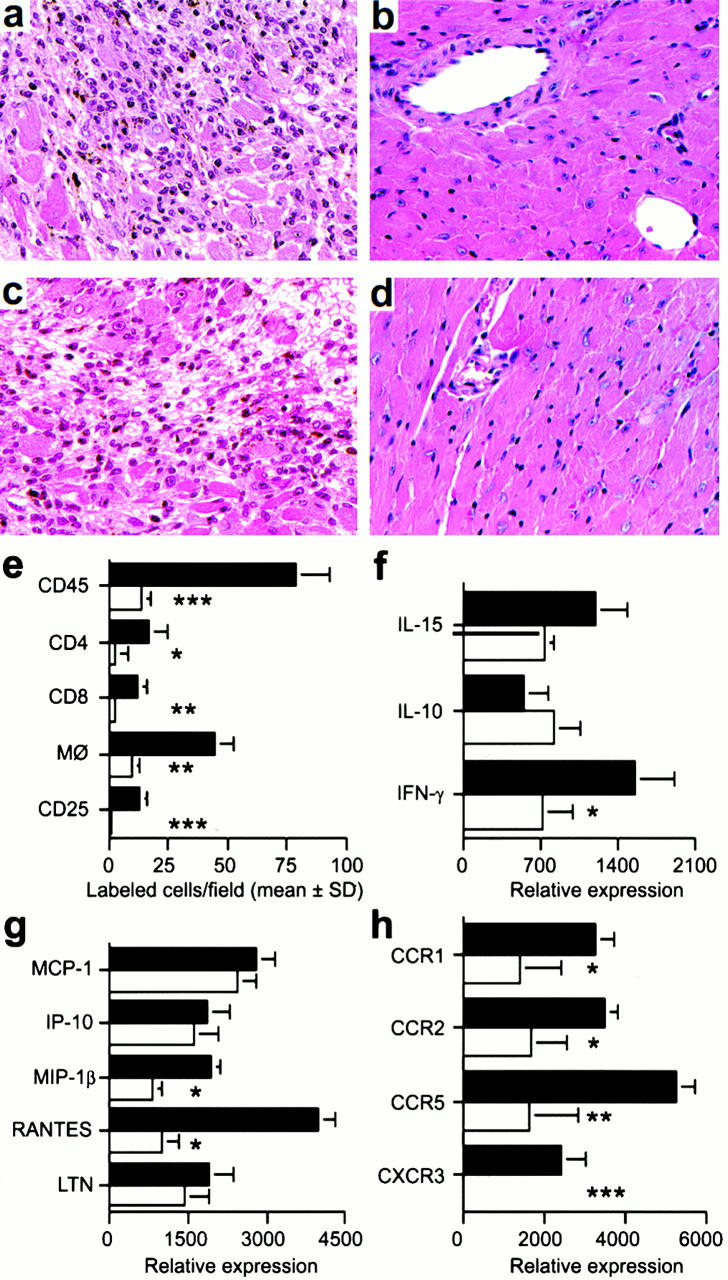
Mechanisms underlying beneficial effects of targeting CXCR3 in allograft recipients. (a) Histology showing acute rejection with extensive mononuclear cell infiltration and myocyte necrosis in CXCR3+/+ recipients (day 7 after transplant). (b) The lack of histologic evidence of rejection in CXCR3−/− recipients (day 7). (c) CXCR3+/+ allograft recipients treated with IgM underwent acute rejection by day 7. (d) Normal appearance of allografts in CXCR3+/+ allograft recipients treated with anti-CXCR3 mAb (day 7). (a–d) Original magnification: ×125. (e) Immunohistology showed significant reduction in recruitment of intragraft CD45+ cells (all leukocytes), CD4 and CD8 T cell subsets, macrophages, and IL-2R+ (CD25+) cells in CXCR3−/− (white bars) versus CXCR3+/+ recipients (black bars) at day 7 after transplant. Data (mean ± SD) are from counting 20 consecutive fields/graft and 3 grafts/group; asterisks indicate significantly reduced cell numbers versus controls (*P < 0.05, **P < 0.01, ***P < 0.005). (f, g, and h) The results of RNase protection assays of the same set of grafts (mean ± SD, four to six grafts/bar), with statistical analysis by the Mann-Whitney U test. CXCR3−/− recipients show decreased mRNA levels of intragraft: (f) IFN-γ (*P < 0.05); (g) MIP-1β and RANTES (both *P < 0.005); and (h) the chemokine receptors CCR1 (*P < 0.05), CCR2 (*P < 0.05), CCR5 (**P < 0.01), and CXCR3 (***P < 0.005). MCP-1, monocyte chemoattractant protein 1; LTN, lymphotactin.
