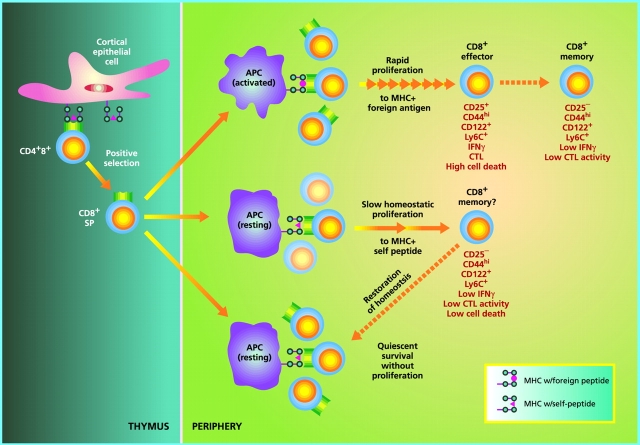
Figure 1.
Regulation of naive CD8+ T cell homeostasis by self-MHC/peptide ligands. In the thymus, immature CD4+8+ double positive cells that interact with low but significant affinity for MHC class I molecules loaded with self-peptides become positively selected and differentiate into mature CD8+ single positive cells. Upon exit to the periphery, CD8+ cells continuously interact with the same self-MHC/peptide ligands or possibly with cross-reactive self-ligands on APCs, and receive low-level signals through the TCR. Under physiological conditions with normal numbers of T cells, such TCR signals are covert, i.e., insufficient to induce entry into cell cycle but adequate to keep cells alive (bottom). However, if the number of T cells drops below a certain level, TCR signals become overtly stimulatory and induce T cells to undergo a slow form of “homeostatic” proliferation in an attempt to restore the size of the T cell pool. During homeostatic proliferation, CD8+ cells resemble resting memory cells in terms of phenotype and their ability to mediate low-level effector function in response to foreign antigens (middle). Upon restoration of the naive T cell pool, homeostatic proliferation stops and some CD8+ clones revert to a naive phenotype, whereas others do not; the basis of this difference is unknown. Generation of “conventional” memory cells through T cell interaction with foreign peptide is qualitatively different in several respects (top). Thus, in contrast to memory cell generation via homeostatic proliferation, production of antigen-specific memory cells (a) requires APC activation (via an adjuvant), (b) involves transition through an activated effector cell phase, (c) is associated with prominent cell death (at the end of the primary response), and (d) leads to long-term expression of memory markers, especially CD44, with little or no reversion to naive phenotype cells. These differences presumably also apply to CD4+ cells (not depicted).