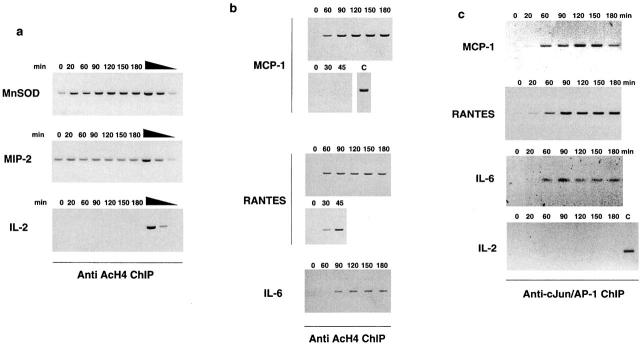
Figure 3.
Stimulus-induced histone H4 hyperacetylation of a subset of NF-κB target promoters precedes NF-κB recruitment. Basal and stimulated levels of histone H4 acetylation were evaluated by ChIP analysis using an anti-AcH4 antiserum (Upstate Biotechnology). Both MnSOD intronic enhancer and MIP-2 promoter were found to display constitutively high levels of histone H4 acetylation (a) which in the case of MnSOD (but not of MIP-2) were further increased upon stimulation. The PCR was performed in the linear range of the amplification as verified using serial dilutions (1, 1:10, 1:100) of the DNA extracted from immunoprecipitation supernatant. The IL-2 gene promoter, which is not active in macrophages, has a constitutively very low level of H4 acetylation, which is not affected by stimulation. Differently from these two NF-κB–dependent promoters, prestimulation histone H4 acetylation levels in the MCP-1, RANTES, and IL-6 promoters were extremely low (b) but increased upon LPS treatment. Recruitment of NF-κB to these promo-ters and accumulation of the corresponding transcripts can be detected only after extensive acetylation has occurred (compare the kinetics shown in b with those in Fig. 2 b). The 0–45-min kinetic comes from a separate experiment. As a positive control (C) for MCP-1 hyperacetylation, the 90-min time point of this experiment is shown. (c) cJun/AP1 recruitment to MCP-1, RANTES, and IL-6 promoters precedes NF-κB recruitment and probably contributes to recruitment of histone acetyltransferases. Anti-cJun ChIP was carried out with a rabbit polyclonal antibody (H79; Santa Cruz Biotechnology, Inc.).