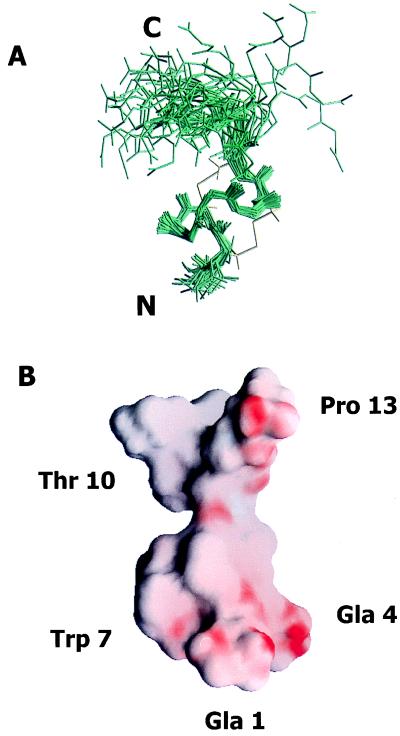
Figure 4.
(A) Three-dimensional structure of ɛ-TxIX. Superimposition of the final 35 structures to the lowest energy conformer of ɛ-TxIX illustrating the (N, Cα, and carbonyl C) backbone atoms of all 13 residues. The peptide backbone pairwise rms deviation is 0.46 ± 0.08 Å over the well defined residues 2 through 10. The cysteine bond arrangement for the lowest energy conformer is identified in yellow. The N and C termini are labeled. (B) Surface view of the electrostatic potential of the lowest energy conformer of ɛ-TxIX illustrating the alignment of the negatively charged residues along one side of the molecule and protruding into the solvent. The surface of the molecule is colored using grasp (41) according to electrostatic potential, scaled from electronegative (red) to electropositive (blue). The Gla, hydroxyproline, bromotryptophan, and glycosylated threonine residues are also labeled.