Abstract
The cytokine interferon (IFN)-γ regulates immune clearance of parasitic, bacterial, and viral infections; however, the underlying mechanisms are poorly understood. Recently, a family of IFN-γ–induced genes has been identified that encode 48-kD GTP-binding proteins that localize to the endoplasmic reticulum of cells. The prototype of this family, IGTP, has been shown to be required for host defense against acute infections with the protozoan parasite Toxoplasma gondii, but not for normal clearance of the bacterium Listeria monocytogenes and murine cytomegalovirus (MCMV). To determine whether other members of the gene family also play important roles in immune defense, we generated mice that lacked expression of the genes LRG-47 and IRG-47, and examined their responses to representative pathogens. After infection with T. gondii, LRG-47–deficient mice succumbed uniformly and rapidly during the acute phase of the infection; in contrast, IRG-47–deficient mice displayed only partially decreased resistance that was not manifested until the chronic phase. After infection with L. monocytogenes, LRG-47–deficient mice exhibited a profound loss of resistance, whereas IRG-47–deficient mice exhibited completely normal resistance. In addition, both strains displayed normal clearance of MCMV. Thus, LRG-47 and IRG-47 have vital, but distinct roles in immune defense against protozoan and bacterial infections.
Keywords: interferon, GTPase, protozoa, bacteria, virus
Introduction
The cytokine IFN-γ plays a central role in host resistance to infection and regulation of the immune system 1 2. IFN-γ increases the expression of over 200 genes that presumably mediate its effects 3; however, for many of these genes, their contribution to IFN-γ–regulated host defense is unknown. Recently, a new family of IFN-γ–induced genes has been identified that are expressed at high levels after infection with many different pathogens. The family contains at least six members that can be separated into two groups based on sequence homology, with group I containing LRG-47 4, IGTP 5 6 and GTPI 7, and group II, IRG-47 8, TGTP/ Mg21 9 10 11, and IIGP 7. These genes encode 47–48-kD GTP-binding proteins that localize to the endoplasmic reticulum of cells 5, and consequently it has been suggested that they may regulate expression or trafficking of proteins of immunological importance 5.
A role in host resistance has been demonstrated for one of these genes, IGTP, through the creation of IGTP-deficient mice 12. These mice display a complete loss of resistance to acute infections of the protozoan parasite Toxoplasma gondii, while maintaining normal defense against Listeria monocytogenes and murine cytomegalovirus (MCMV). In contrast, IFN-γ–deficient mice display decreased resistance to all three agents 13 14 15 16. Therefore, IGTP is an essential element of IFN-γ–induced host defense, but it only mediates the antimicrobial effects of the cytokine against certain pathogens. No in vivo data exist regarding the function of the other proteins in this family.
In these studies, we used gene targeting to generate mice that lack expression of LRG-47 and IRG-47, representatives of the two subgroups of the IGTP protein family. The resulting phenotypes of the LRG-47– and IRG-47–deficient mice demonstrate that although each is essential for normal host resistance, each plays a distinct role in IFN-γ–induced clearance of intracellular pathogens.
Materials and Methods
LRG-47 and IRG-47 Gene Targeting.
The LRG-47 targeting vector was constructed from LRG-47 gene fragments that were isolated from a 129SvJ mouse library (Stratagene) as contiguous 5- and 3-kb XbaI fragments containing the entire LRG-47 protein coding region (sequence data are available from GenBank/EMBL/DDBJ under accession no. U19119) and a single intron within the coding region. In the targeting vector, a 0.95-kb SpeI-XbaI portion of the 5-kb fragment, including 0.7-kb of the protein coding region, was deleted and replaced with pGKneoBpA that served as a positive selective marker. These sequences were then flanked by pGKtkBpA 17 18, a negative selective marker.
The IRG-47 targeting vector was constructed from IRG-47 gene fragments that were isolated from a 129SvJ library as a 3.0-kb SacI fragment containing the 5′ portion of the protein coding region (GenBank M63630) and upstream sequences, and a 5.5-kb XbaI fragment containing the 3′ untranslated region (GenBank M63630) and downstream sequences. The targeting vector was created by separating a 2-kb HindIII-NcoI portion of the 3.0-kb SacI fragment, and the entire 5.5-kb XbaI fragment, with pGKneoBpA 17 18, which in effect deleted the complete protein coding region of the gene. These sequences were then flanked with pGKtkBpA 17 18.
The targeting vectors were electroporated into CJ7 embryonic stem cells 17 18, and homologous recombinants were selected by Southern blotting of EcoRI-restricted DNA with an LRG-47 probe (a 0.5-kb BglII of the LRG-47 cDNA) or an IRG-47 probe (a 0.5-kb SacI-HindIII fragment of the 3.0-kb SacI IRG-47 genomic clone). Using the targeted cells and established procedures, LRG-47– and IRG-47–deficient mice were generated on a C57BL/6 × 129SvJ genetic background 17 18. All experiments were performed with 1–4-mo-old mice, and the mice were housed in a specific pathogen-free facility.
IFN-γ–deficient mice on a C57BL/6 background were obtained from the National Institute of Allergy and Infectious Disease Facility at Taconic Farms, Inc.
Protein and RNA Analyses.
For Western blotting, protein lysates were isolated from cells or tissues, separated by 10% SDS-PAGE, and blotted as described previously 6. Rabbit polyclonal anti–LRG-47 antisera recognized the internal LRG-47 peptide sequence YNTGSSRLPEVSRSTE 4, and rabbit polyclonal anti–IRG-47 antisera recognized the COOH-terminal IRG-47 sequence DDAKHLLRKIDTVNVA 8.
For Northern blot analysis, 15 μg total RNA samples were separated on 1.2% agarose/formaldehyde gels and blotted with labeled probes as described previously 6. The probes included a human glyceraldehyde phosphate dehydrogenase probe isolated as a 1.2-kb fragment of pHcGAP 19, a mouse IGTP 3′ untranslated region probe isolated as a 0.28-kb EcoRI fragment of the IGTP cDNA 6, a mouse LRG-47 cDNA probe isolated as a 1.4-kb KpnI fragment of the LRG-47 cDNA (GenBank U19119), and a mouse IRG-47 3′ untranslated region probe corresponding to bases 1,374 to 1,625 of the IRG-47 cDNA (GenBank M63630) that was isolated using the polymerase chain reaction.
T. gondii Infection.
Mice were injected intraperitoneally with 0.5 ml PBS containing 20 cysts of the avirulent ME49 strain of T. gondii, that had been prepared from the brains of infected C57BL/6 mice. The mice were monitored daily.
For ex vivo cytokine analysis, single-cell spleen suspensions and peritoneal exudate cells were isolated from infected mice, and contaminating red cells were removed using ACK lysing buffer (Bio Whittaker). The spleen cells and peritoneal exudate cells (PECs) were then cultured in 96-well plates, at 8 × 105 and 4 × 105 cells per well respectively, in 200 μl RPMI medium (Life Technologies) supplemented with 10% (vol/vol) fetal bovine serum (Life Technologies). In some cases, the cell cultures were stimulated with 10 μg/ml plate-bound anti-CD3 (BD PharMingen) or 10 μg/ml STAg (soluble tachyzoite antigen), which had been prepared from sonicated RH parasites as described previously 20. Conditioned media were collected 72 h later for determination of IFN-γ and IL-12 p40 levels using sandwich ELISA as described previously 13.
Sera were prepared from blood that was collected at the time of sacrifice, allowed to clot, and then centrifuged at 6,000 rpm for 10 min.
L. monocytogenes Infection.
The mice were inoculated intraperitoneally with 1,000 CFU of the L. monocytogenes EGD strain (provided by Dr. K Elkins, U.S. Food and Drug Administration, Bethesda, MD). Health and survival of the mice were monitored daily for at least 14 d. For experiments involving the measurement of bacterial loads in the spleen and liver, the tissues were isolated 3 d after inoculation. Bacterial counts were then determined by homogenizing portions of the organs in PBS, and plating serial dilutions of the homogenates on LB agar plates. Colony counts were determined the following day, and the total bacterial load per organ was calculated.
MCMV Infection.
Salivary gland MCMV stocks were generated by inoculating C57BL/6-129SvImJ mice intraperitoneally with 104 PFU of the Smith MCMV strain (American Type Culture Collection VR-194). At 11 d after inoculation, the salivary glands were isolated and homogenized in 10% (vol/vol) fetal bovine serum (FBS; Hyclone)/DMEM (Life Technologies). Viral stocks were titered by infecting confluent lawns of primary embryonic fibroblasts in 6-well tissue culture plates with dilutions of the viral lysates. The infected cells were overlaid with 1% agar (wt/vol) in 2% FBS/DMEM and then incubated 4–7 d. PFUs were identified microscopically.
To assess host restriction of MCMV infection, mice were inoculated intraperitoneally with 5 × 104 PFU MCMV in 0.5 ml 10% FBS/DMEM. 3 d later, spleen and liver samples were isolated and homogenized in the same medium, and used for PFU determination.
Results and Discussion
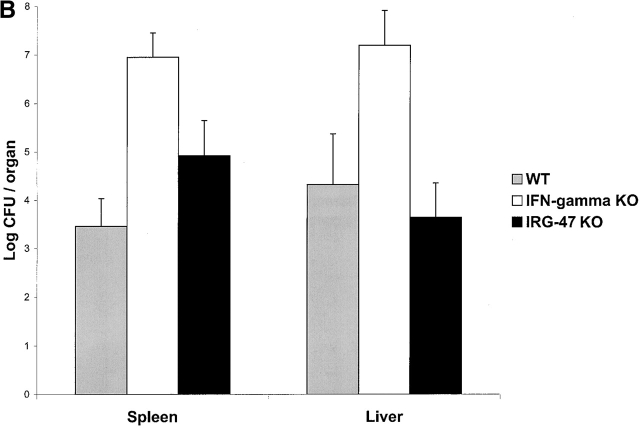
LRG-47 and IRG-47 belong to a family of IFN-γ–induced proteins whose expression is increased to very high levels in mice after infection with numerous pathogens, including T. gondii, L. monocytogenes, and MCMV (Fig. 1). To determine the roles that LRG-47 and IRG-47 might play in mediating IFN-γ–stimulated resistance to these pathogens, we used gene targeting to create mice that lacked expression of the two proteins (Fig. 2), and then assessed their ability to restrict the three infections. In absence of infection, the LRG-47 and IRG-47–deficient mice displayed no obvious abnormalities; they were produced in normal numbers, and necropsies revealed no major alterations in tissue architecture (data not shown). In addition, FACS® analysis of splenocytes from adult mice revealed no changes in the development of T cell, B cell, macrophage, and NK cells (data not shown). However, when challenged with the three model pathogens, the mice displayed profound, but selective, losses in host resistance.
Figure 1.
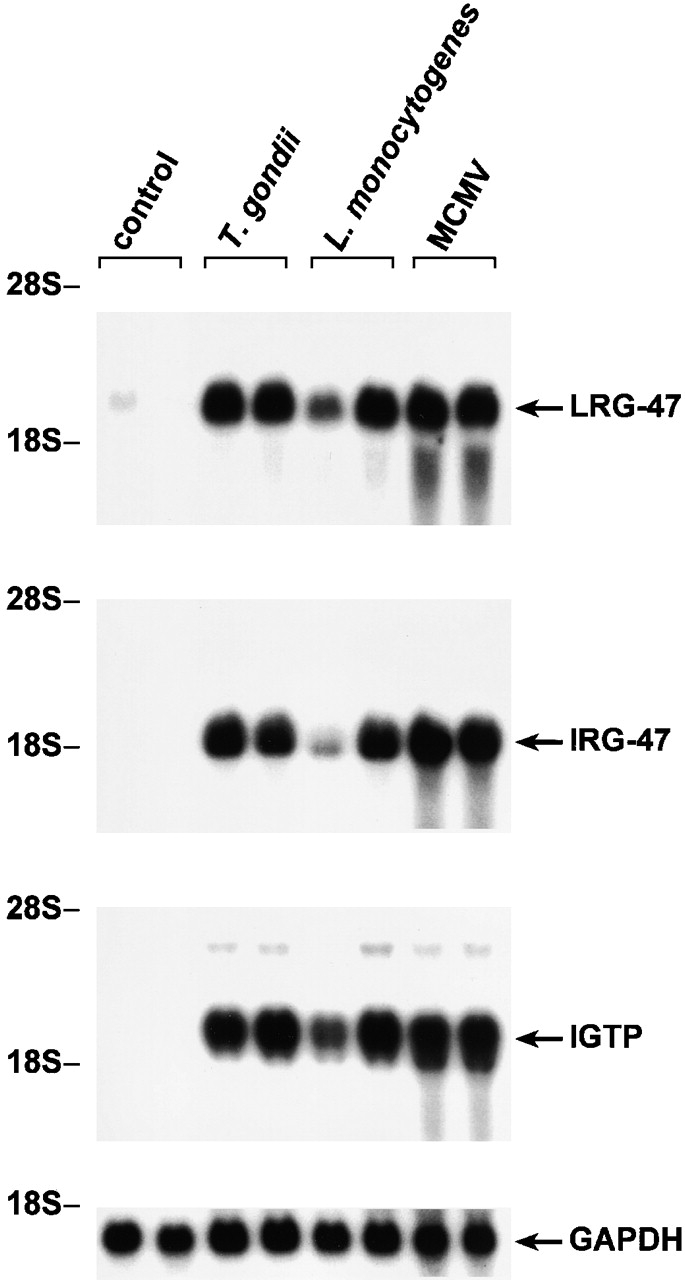
Induction of LRG-47 and IRG-47 expression in response to different pathogens. Pairs of mice were inoculated as indicated with 20 cysts T. gondii for 8 d, 1,000 CFU L. monocytogenes for 5 d, or 5 × 104 PFU MCMV for 36 h, or were left uninfected (control). Total RNA was prepared from liver and used for sequential Northern blotting with LRG-47, IRG-47, IGTP, and GAPDH probes. Positions of the major ribosomal RNA species are indicated.
Figure 2.
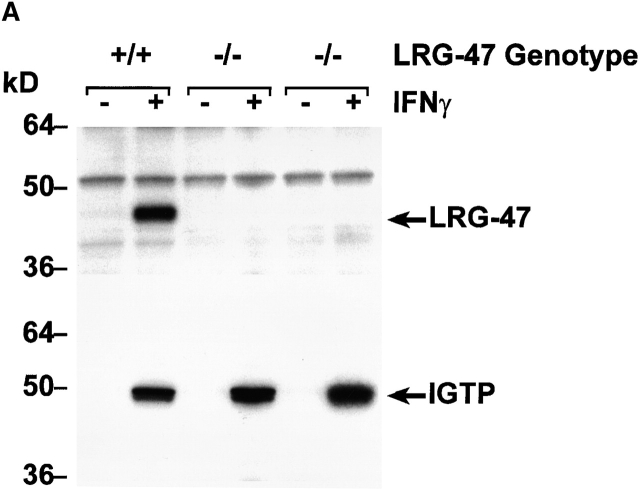
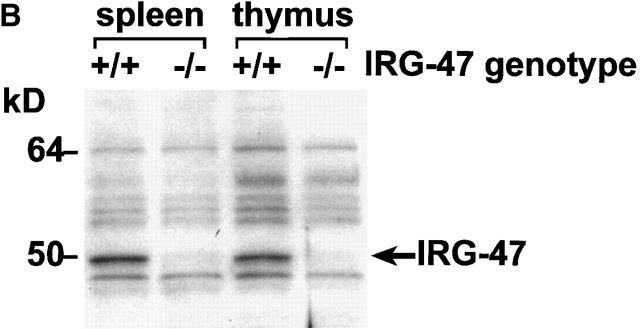
Gene targeting to create LRG-47– and IRG-47–deficient mice. As described in detail in the Materials and Methods, standard gene targeting techniques were used to generate mice that lack production of LRG-47 and IRG-47. Western blotting was then used to verify absence of protein expression. (A) Embryonic fibroblasts from wild-type (+/+) or LRG-47–deficient (−/−) mice were exposed to control conditions or 100 U/ml IFN-γ for 15 h. Lysates were prepared from the cells, resolved by 10% SDS-PAGE, and used for sequential Western blotting with anti–LRG-47 and anti-IGTP antisera. (B) Spleen and thymus were isolated from wild-type (+/+) or IRG-47–deficient (−/−) mice. Lysates were prepared, resolved by 10% SDS-PAGE, and used for Western blotting with anti–IRG-47 antisera. Expression in IRG-47–deficient fibroblasts could not be assessed because of cross-reacting bands in the fibroblast lysates that were recognized by the anti–IRG-47 antisera.
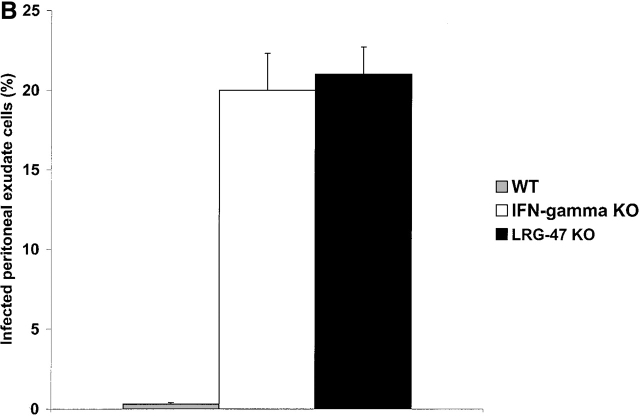
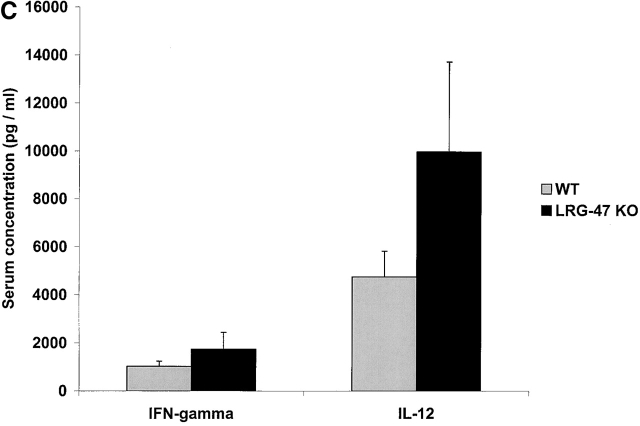
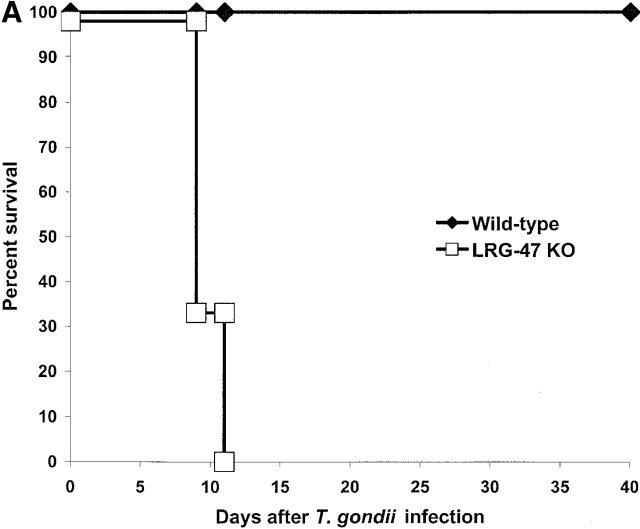
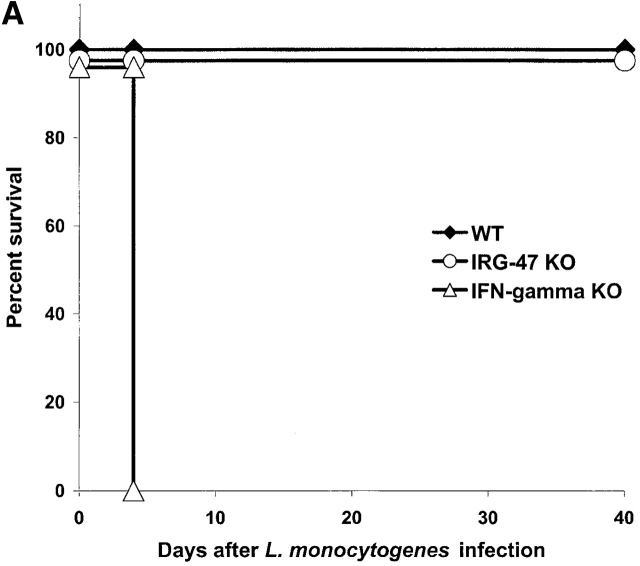
We began by examining the susceptibility of the mice to the intracellular protozoan parasite T. gondii. Infection with T. gondii is characterized by an acute phase in which the rapidly proliferating form, or tachyzoite, disseminates throughout the host, followed by a chronic phase in which a dormant form, or bradyzoite, inhabits mainly central nervous tissue and muscle 21. IFN-γ is absolutely required for control of both phases 13 21. After invasion of the host cell, T. gondii resides in a parasitophorous vacuole that resists interaction with the endocytic machinery of the cell, and consequently provides the organism with a safe environment in which to replicate 22. LRG-47–deficient, IRG-47–deficient, and wild-type mice were inoculated intraperitoneally with 20 cysts of T. gondii, and their responses were assessed. Similar to what has been shown previously for IGTP-deficient mice 12, LRG-47–deficient mice displayed a complete loss of resistance to the parasite during the acute phase of infection, dying between days 9 and 11 after inoculation (Fig. 3 A), the same time frame in which IFN-γ–deficient mice succumb to the infection 13. When the mice were examined 5 d after infection, there was a marked increase in the number of T. gondii infected cells in the peritoneum of the LRG-47–deficient mice (Fig. 3 B), comparable to that seen in IFN-γ–deficient mice 13, which indicated that the mice were not able to suppress replication of the parasite. Also at 5 d after infection, IFN-γ and IL-12 production was robust (Fig. 3 C), indicating that loss of resistance was not due to lack of production of the two cytokines. The modestly elevated levels of the two cytokines in the LRG-47–deficient mice were probably due to the unattenuated replication of the parasite. Importantly, decreased resistance to T. gondii in the LRG-47–deficient mice was not a result of decreased IGTP production, given that IGTP levels in splenocytes increased to high levels after T. gondii infection in vivo (data not shown), and IGTP expression was increased normally in LRG-47–deficient fibroblast cultures after stimulation with IFN-γ (Fig. 2 A). Therefore, IGTP and LRG-47 are independent factors that are both critically important for normal clearance of acute T. gondii infections.
Figure 3.
Acute loss of resistance to T. gondii in LRG-47–deficient mice. The indicated strains were inoculated intraperitoneally with 20 cysts T. gondii, and their ability to restrict the infection was assessed. (A) Wild-type (n = 6) and LRG-47–deficient mice (n = 6) were monitored for their survival for 40 d. KO, knockout. (B) Peritoneal exudate cells from wild-type (WT; n = 3), IFN-γ–deficient (n = 3), and LRG-47–deficient mice (n = 4) were isolated at 5 d after infection, and the presence of intracellular T. gondii was determined microscopically. (C) Sera were isolated from wild-type (n = 2) and LRG-47–deficient mice (n = 4) at 5 d after infection and used for IFN-γ and IL-12 determination by ELISA. A–C are representative results of two experiments.
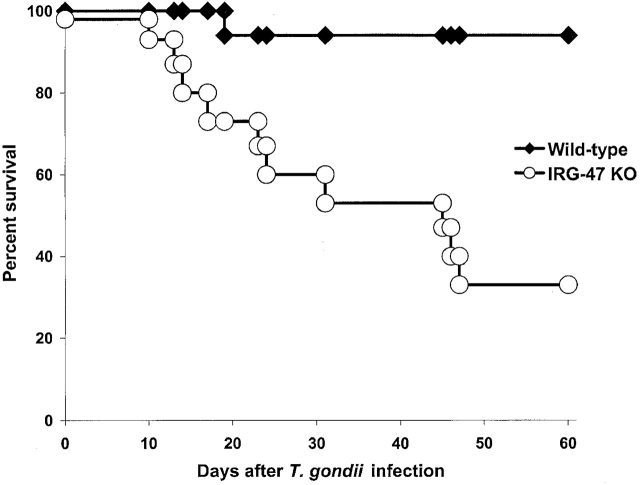
In contrast to the IGTP-deficient and LRG-47–deficient mice, IRG-47–deficient mice displayed only marginally reduced resistance to T. gondii (Fig. 4). After challenge with 20 cysts of the parasite, 67% of the IRG-47–deficient mice died, with death occurring mainly during the chronic phase between days 10 and 47 after infection (Fig. 4). The burden of T. gondii cysts in the brains of IRG-47–deficient mice correlated with the partial decrease in survival in the chronic phase of infection: at 33 d after infection there were 2,575 ± 1,237 cysts per brain in IRG-47–deficient mice vs. 3,300 ± 1,387 in wild-type mice, while at 57 d there were 1,447 ± 654 in IRG-47–deficient mice vs. 913 ± 228 in wild-type mice. IFN-γ production was normal in IRG-deficient mice, with peritoneal exudate cells from IRG-47–deficient mice producing 5,164 ± 1,153 pg/ml IFN-γ after exposure to soluble T. gondii tachyzoite extract, STAg, compared with 4,558 ± 3,011 pg/ ml from wild-type cells. Thus, as opposed to LRG-47 and IGTP, IRG-47 plays only a modest role in restricting T. gondii infections, and this does not become apparent until the chronic phase of infection. The differing responses of the mice to T. gondii infections reflect the subdivision of the IGTP protein family into two subfamilies based on primary sequence homology, with one subfamily containing LRG-47 and IGTP, and the second IRG-47.
Figure 4.
Marginal loss of resistance to T. gondii in IRG-47–deficient mice. Wild-type (n = 17) and IRG-47–deficient mice (n = 15) were monitored for their survival for 60 d. Shown are the cumulative results of two experiments. KO, knockout.
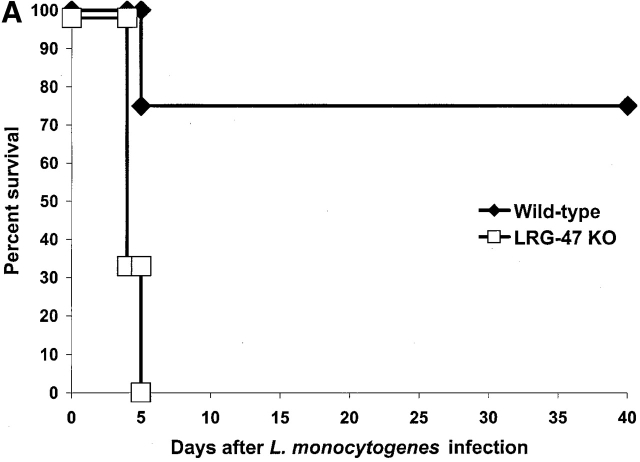
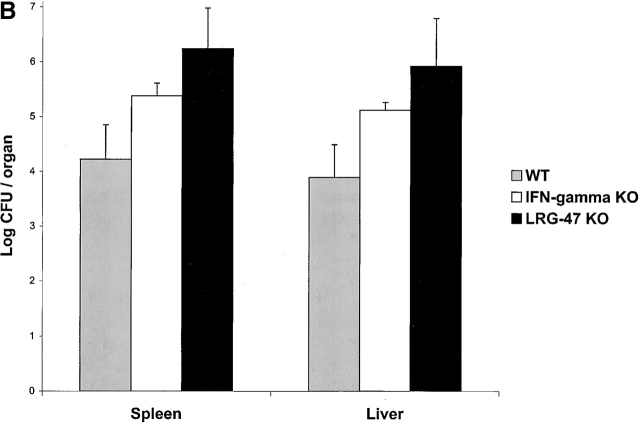
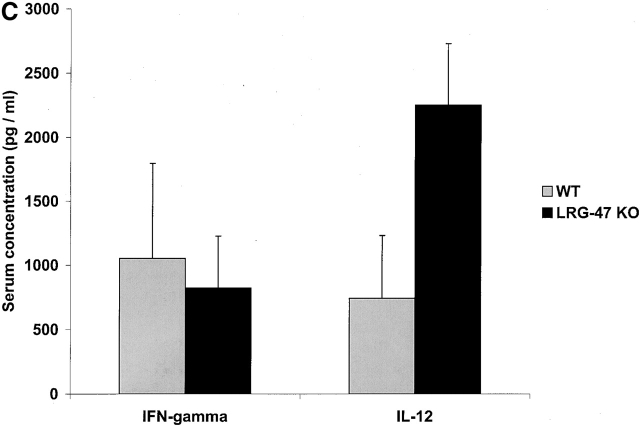
Next, the mice were challenged with L. monocytogenes, a gram-positive bacterium that produces an acute infection. L. monocytogenes is an intracellular bacterium that resides in a vacuole briefly after penetration of the host cell, but thereafter lyses the vacuole to release itself into the cytosol where it replicates 23. IFN-γ signaling is critical for normal restriction of L. monocytogenes 15, but IGTP is not required for normal resistance 12. To determine whether LRG-47 and IRG-47 are required, mice that lack their expression were infected with 1,000 CFUs of L. monocytogenes and then monitored for their responses (Fig. 5 and Fig. 6). Surprisingly, LRG-47–deficient mice succumbed rapidly, displaying uniform death by 5 d, paralleling that seen in IFN-γ–deficient mice 12 15 (Fig. 5 A). Decreased survival in the LRG-47–deficient mice correlated with greatly increased bacterial burdens in the liver and spleen at 3 d after infection (Fig. 5 B). Also in the LRG-47–deficient mice, IFN-γ production was equivalent to that of wild-type mice, while IL-12 production was elevated (Fig. 5 C).
Figure 5.
Marked loss of resistance to L. monocytogenes in LRG-47–deficient mice. The indicated strains were inoculated intraperitoneally with 1,000 CFU L. monocytogenes, and their ability to restrict the infection was assessed. (A) Wild-type (n = 4) and LRG-47–deficient mice (n = 6) were monitored for their survival for 40 d. KO, knockout. (B) Spleen and liver of wild-type (WT; n = 7), IFN-γ–deficient (n = 7), and LRG-47–deficient mice (n = 9) were isolated at 3 d after infection, and the numbers of bacteria present was determined. Statistical analysis for wild-type vs. LRG-47–deficient mice: spleen, P = 0.003; liver, P = 0.05. (C) Sera were isolated from wild-type (n = 3) and LRG-47–deficient mice (n = 5) at 3 d after infection and used for IFN-γ and IL-12 determination by ELISA. A–C are representative results from two experiments.
Figure 6.
Normal resistance to L. monocytogenes in IRG-47–deficient mice. (A) Wild-type (WT; n = 6), IFN-γ–deficient (n = 6), and IRG-47–deficient mice (n = 6) were monitored for their survival for 40 d. KO, knockout. (B) Spleen and liver of wild-type (n = 4), IFN-γ–deficient (n = 3), and IRG-47–deficient mice (n = 5) were isolated at 3 d after infection, and the number of bacteria present was determined. Statistical analysis for wild-type vs. IRG-47–deficient mice: spleen, P = 0.01; liver, P = 0.28. The slight increase in the splenic bacterial loads of IRG-47–deficient mice, compared with that of wild-type mice, was not seen in a second experiment. A and B are representative results from two experiments.
In contrast to the LRG-47–deficient mice, the IRG-47–deficient mice showed no adverse effects and 100% survival after L. monocytogenes infection (Fig. 6 A). In addition, the bacterial loads in the liver and spleen of IRG-47–deficient mice were equivalent to those in wild-type mice (Fig. 6 B). Thus, LRG-47 plays a central role in mediating IFN-γ–induced clearance of L. monocytogenes, whereas IRG-47 and IGTP 12 are dispensable.
Finally, the response of the mice to MCMV was characterized. MCMV is a double-stranded DNA herpes virus that, in an immunocompetent host, establishes a latent infection; however, absence of IFN-γ signaling leads to an acute infection with increased viral loads and mortality 24. LRG-47–deficient and IRG-47–deficient mice were inoculated with MCMV, and at 3 d after inoculation, the loads of MCMV in tissues of the mice were determined. In both spleen and liver, comparable numbers of viral plaque-forming units were detected in wild-type, LRG-47–deficient, and IRG-47–deficient mice (data not shown). Similar results have been reported previously for IGTP-deficient mice 12. Therefore, LRG-47, IRG-47, and IGTP are not critical factors for defense against MCMV.
Taken together, our data demonstrate that LRG-47 and IRG-47 are members of a protein family that plays a central role in the IFN-γ–mediated clearance of infection, with each member of the family supporting host resistance to a different spectrum of pathogens (Table ). Because each of the genes is expressed at high levels after infection, their differential roles in host resistance are likely to relate to distinct molecular functions. Although LRG-47, IRG-47, and the related proteins are expressed in hematopoietic and nonhematopoietic cells, there is no evidence to suggest that they modulate classical immune functions, given that LRG-47–, IRG-47–, and IGTP-deficient macrophages produce normal levels of nitric oxide and TNF-α (data not shown), and IGTP-deficient CD8 T cells and NK cells exhibit normal cytotoxic functions 12. Rather, it seems likely that the proteins act within the host cell to undermine survival of invading pathogens. Considering their purported roles as regulators of protein expression or trafficking 5, it is possible that LRG-47 and IRG-47 may function by altering trafficking to host cell vacuoles that contain T. gondii or L. monocytogenes, thereby affecting vacuole acidification and maturation, and compromising survival of the pathogen. Parallels can be drawn with some members of the rab family of GTP-binding proteins that are present in the endosomal compartment, as in vitro data suggest that they may regulate vesicular trafficking to intracellular pathogens 25 26 . The studies presented here suggest that another family of GTP-binding proteins in a compartment more distal to the vacuole, the endoplasmic reticulum (ER), plays a critical role in governing the fate of microbes in IFN-γ–activated cells.
Table 1.
Differential Loss of Host Resistance to Representative Pathogens among Mice Lacking IGTP Family Proteins
| T. gondii | L. monocytogenes | MCMV | |
|---|---|---|---|
| LRG-47 KO | S (acute) | S | R |
| IRG-47 KO | S (chronic) | R | R |
| IGTP KO | S (acute) | R | R |
| IFN-γ | S (acute) | S | S |
Acknowledgments
We are grateful to Drs. John D. Hamilton and S. Charles Henry for supplying the MCMV stock and protocols, and to Sara Hieny and Pat Caspar for assistance with T. gondii and L. monocytogenes assays.
This work was supported in part by National Institutes of Health grant AG11268, and by John A. Hartford Foundation grant 990339.
Footnotes
Abbreviations used in this paper: FBS, fetal bovine serum; MCMV, murine cytomegalovirus.
References
- Stark G.R., Kerr I.M., Williams B.R.G., Silverman R.H., Shreiber R.D. How cells respond to interferons. Annu. Rev. Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- Boehm U., Klamp T., Groot M., Howard J.C. Cellular responses to interferon-γ. Annu. Rev. Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- Der S.D., Zhou A., Williams B.R.G., Silverman R.H. Identification of genes differetially regulated by interferon α, β, or γ using oligonucleotide arrays. Proc. Natl. Acad. Sci. USA. 1998;95:15623–15628. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorace J.M., Johnson R.J., Howard D.L., Drysdale B.E. Identification of an endotoxin and IFN-γ-inducible cDNApossible identification of a novel protein family. J. Leukoc. Biol. 1995;58:477–484. doi: 10.1002/jlb.58.4.477. [DOI] [PubMed] [Google Scholar]
- Taylor G.A., Stauber R., Rulong S., Hudson S.E., Pei V., Pavlakis G.N., Resau J.H., Vande Woude G.F. The inducibly expressed GTPase (IGTP) localizes to the endoplasmic reticulum independently of GTP binding. J. Biol. Chem. 1997;272:10639–10645. doi: 10.1074/jbc.272.16.10639. [DOI] [PubMed] [Google Scholar]
- Taylor G.A., Jeffers M., Largaespada D.A., Jenkins N.A., Copeland N.G., Vande Woude G.F. Identification of a novel GTPase, the inducibly expressed GTPase (IGTP), that accumulates in response to interferon-gamma. J. Biol. Chem. 1996;271:20399–20405. doi: 10.1074/jbc.271.34.20399. [DOI] [PubMed] [Google Scholar]
- Boehm U., Guethlein L., Klamp T., Ozbek K., Schaub A., Futterer A., Pfeffer K., Howard J.C. Two families of GTPases dominate the cellular response to IFNγ. J. Immunol. 1998;161:6715–6723. [PubMed] [Google Scholar]
- Gilly M., Wall R. The IRG-47 gene is IFN-γ induced in B cells and encodes a protein with GTP-binding motifs. J. Immunol. 1992;148:3275–3281. [PubMed] [Google Scholar]
- Carlow D.A., Marth J., Clark-Lewis I., Teh H.-S. Isolation of a gene encoding a developmentally regulated T cell-specific protein with a guanidine nucleotide triphosphate-binding motif. J. Immunol. 1995;154:1724–1734. [PubMed] [Google Scholar]
- LaFuse W.P., Brown D., Castle L., Zwilling B.S. Cloning and characterization of novel cDNA that is IFN-γ-induced in mouse peritoneal macrophages and encodes a GTP-binding protein. J. Leukoc. Biol. 1995;57:477–483. doi: 10.1002/jlb.57.3.477. [DOI] [PubMed] [Google Scholar]
- Carlow D.A., Teh S.-J., Teh H.-S. Specific antiviral activity demonstrated by TGTP, a member of a new family of interferon-induced GTPases. J. Immunol. 1998;161:2348–2355. [PubMed] [Google Scholar]
- Taylor G.A., Collazo C.M., Yap G.S., Nguyen K., Gregorio T., Taylor L.S., Eagleson B., Secrest L., Southon E., Reid S.W. Pathogen specific loss of host resistance in mice lacking the interferon-γ-inducible gene IGTP. Proc. Natl. Acad. USA. 2000;97:751–755. doi: 10.1073/pnas.97.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharton-Kersten T.M., Wynn T.A., Denkers E.Y., Bala S., Grunvald E., Hieny S., Gazzinelli R.T., Sher A. In the absence of endogenous IFN-γ, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J. Immunol. 1996;157:4045–4054. [PubMed] [Google Scholar]
- Dalton D.K., Pitts-Meek S., Keshav S., Figari I.S., Bradley A., Stewart T. Multiple defects in immune cell function in mice with disrupted interferon-γ genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- Huang S., Hendriks W., Althage A., Hemmi S., Bluethmann H., Kamijo R., Vilcek J., Zinjernagel R.M., Aguet M. Immune response in mice that lack the interferon-γ receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- Presti R.M., Pollock J.L., Dal Canto A.J., O'Guin A.K., Virgin H.W. Interferon-γ regulates acute and latent murine cytomegalovirus infection and chronic disease of the great vessels. J. Exp. Med. 1998;188:577–588. doi: 10.1084/jem.188.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonin A., Reid S.W., Tessarollo L. Gene knockoutsIsolation microinjection and transfer of mouse blastocysts. Methods Mol. Biol. 2001;158:121–134. doi: 10.1385/1-59259-220-1:121. [DOI] [PubMed] [Google Scholar]
- Tessarollo L. Gene knockoutsmanipulating mouse embryonic stem cells. Methods Mol. Biol. 2001;158:47–63. doi: 10.1385/1-59259-220-1:47. [DOI] [PubMed] [Google Scholar]
- Tso J.Y., Sun X.H., Kao T.H., Reece K.S., Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAsgenomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985;13:2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunvald E., Chiaramonte M., Hieny S., Wysocka M., Trincieri G., Vogel S.N., Gazinelli R.T., Sher A. Biochemical characterization and protein kinase C dependency of monokine-inducing activities of Toxoplasma gondii . Infect. Immun. 1996;64:2010–2018. doi: 10.1128/iai.64.6.2010-2018.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap G.S., Sher A. Cell-mediated immunity to Toxoplasma gondiiinitiation, regulation, and effector function. Immunobiology. 1999;201:240–247. doi: 10.1016/S0171-2985(99)80064-3. [DOI] [PubMed] [Google Scholar]
- Sibley L.D. Interactions between Toxoplasma gondii and its mammalian host cells. Semin. Cell Biol. 1993;4:335–344. doi: 10.1006/scel.1993.1040. [DOI] [PubMed] [Google Scholar]
- Cossart P., Lecuit M. Interactions of Listeria monocytogenes with mammalian cells during entry and actin-based movementbacterial factors, cellular ligands and signaling. EMBO J. 1998;17:3797–3806. doi: 10.1093/emboj/17.14.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange J.S., Wang B., Terhorst C., Biron C.A. Requirement for natural killer cell–produced interferon-γ in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J. Exp. Med. 1995;182:1045–1056. doi: 10.1084/jem.182.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Dominguez C., Barbier A.M., Beron W., Wandinger-Ness A., Stahl P.D. Phagocytosed live Listeria monocytogenes influences Rab5-regulated in vitro phagosome-endosome fusion. J. Biol. Chem. 1996;271:13834–13843. doi: 10.1074/jbc.271.23.13834. [DOI] [PubMed] [Google Scholar]
- Meresse S., Steele-Mortimer O., Finlay B.B., Gorvel J.-P. The rab7 GTPase controls the maturation of Salmonella typhimurium-containing vacuoles. EMBO J. 1999;18:4394–4403. doi: 10.1093/emboj/18.16.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]