Recognition of antigen by CD4+ T cells requires presentation of short peptide fragments in the context of heterodimeric MHC class II molecules 1 2. Antigen presentation by MHC class II molecules is pivotal to the induction of adaptive immune responses, peripheral tolerance, and central tolerance, as well as being required for CD4+ T cell survival 1 2 3. Constitutive expression of MHC class II molecules is restricted to APCs: B cells, dendritic cells and macrophages; and cortical thymic epithelial cells (cTECs), where MHC class II molecules mediate positive selection of CD4+ T cells 4. The expression of MHC class II on APCs can be further upregulated in response to inflammatory mediators such as IFN-γ and LPS. These stimuli are also able to induce MHC class II expression on other cell types, e.g., epithelial cells and endothelial cells. However, the precise role of inducible MHC class II expression on non-APCs remains unclear, although it has been implicated in many autoimmune diseases, allograft rejection, and clearance of pathogens from the body 5 6 7.
Regulation of MHC class II expression at the transcriptional level is complex, and the class II transactivator (CIITA) is a non–DNA-binding transcriptional activator that plays a key role in this process 8 9. CIITA was first identified in complementation studies as one of the genes responsible for bare lymphocyte syndrome in humans, a congenital disease in which patients lack both constitutive and inducible expression of MHC class II molecules 10. CIITA is thought to mediate transcriptional activation by interacting with a large number of DNA-binding proteins to form a complex that initiates transcription 8 9. These proteins include RFX5, RFXANK, CREB, and NF-Y, and they bind specific regions in the MHC class II promoter, known as the W, X, and Y boxes 8 9. It is believed that CIITA provides the specificity for the expression of MHC class II, as the other transcription factors present in the initiating complex are expressed ubiquitously 8 9.
The role of CIITA in the regulation of MHC class II expression has been further dissected in many in vitro and in vivo studies. Transfection of CIITA into cell lines and primary cells that normally lack MHC class II expression has been shown to be sufficient to induce MHC class II expression, while in CIITA-deficient animals, MHC class II mRNA is barely detectable and cell surface expression is absent on the majority of cells 11 12 13. Further evidence indicating the importance of CIITA in regulating MHC class II comes from the observation that the level of expression of MHC class II correlates directly with that of CIITA 14. In addition, the loss of MHC class II expression on B cells as they differentiate into plasma cells has been shown to be concomitant with a loss of CIITA 15, and the absence of MHC class II expression in trophoblasts has been reported to be accompanied by a lack of CIITA 13. Taken together, these observations have lead to the conclusion that CIITA acts as the master regulator of MHC class II expression 8 9.
The major mechanism by which CIITA expression is regulated is transcriptional. Four independent promoters of the CIITA gene (Mhc2ta) have been identified in humans and three in mice 16. Initiation of transcription from each promoter generates distinct gene products, each differing at their NH2 terminus, the significance of which is unknown at present 8 9. Differential promoter usage has been observed in distinct cell types, with promoter I (pI) being highly specific for DCs, B cells primarily using promoter III (pIII), and promoter IV (pIV) mediating IFN-γ–induced upregulation of CIITA in APCs as well as other cell types 16. This specificity of promoter usage is not complete, however, as pIII transcripts have been observed in some populations of DCs, and although pIV is the most important promoter for IFN-γ–induced upregulation, pIII has also been shown to be IFN-γ responsive 17.
Studies of the molecular mechanisms regulating the responsiveness of pIV to IFN-γ have provided evidence for the cooperative involvement of a number of transcription factors, in particular, the IFN-γ induced transcription factors signal transducer and activator of transcription-1 (STAT-1) and IFN regulatory factor-1 (IRF-1) and the constitutively expressed upstream stimulating factor-1 (USF-1) 17 18 19. However, little is known about the biological significance of IFN-γ–induced upregulation of MHC class II on non-APCs, as previous studies have been unable to separate this from the expression of MHC class II by APCs 12 20. In this issue, Waldburger et al. 21 circumvent this problem by generating mice lacking pIV of CIITA, in which the expression of MHC class II on the surfaces of APCs is segregated from its IFN-γ–inducible expression on nonhematopoietic cells. In addition, these mice provide the first definitive evidence that differential CIITA promoter usage does indeed play an important physiological role.
Perhaps the most surprising observation of Waldburger et al. 21 is that constitutive expression of MHC class II on cTECs is eliminated in pIV-deficient animals. MHC class II expression on cTECs has been shown to be independent of IFN-γ signaling, as CD4+ T cell positive selection is not impaired in IFN-γ–deficient or IFN-γ receptor–deficient animals 22 23. This suggests that the pathway leading to the initiation of transcription from pIV in cTECs must differ from that induced by IFN-γ in other nonhematopoietic cells. Further evidence for this hypothesis comes from the observation that positive selection of CD4+ T cells occurs normally in animals deficient in either of the two transcription factors shown to be responsible for initiating pIV transcription in response to IFN-γ, STAT-1, or IRF-1 24 25. However, IRF-1 has been shown to initiate pIV transcription independently of STAT-1 17, and therefore, analysis of positive selection in IRF-1 × STAT-1 double-deficient animals would be necessary to completely rule out any role for these transcription factors in regulating constitutive MHC class II expression on cTECs.
Thymic stromal cell lines lack MHC class II expression, and freshly isolated cTECs lose MHC class II expression if cultured as a cell suspension or a two-dimensional monolayer. However, reexpression can be induced on these cells by exposure to IFN-γ, thus indicating that the pathway mediating IFN-γ–induced CIITA expression can be functional in cTECs 26 27 28. Interestingly, MHC class II expression on cTECs is maintained in the absence of IFN-γ if they are cultured as three-dimensional reaggregates known as reaggregate thymus organ cultures (RTOCs) 29. This implies that MHC class II expression is maintained on cTECs by a signal or stimulus, distinct from IFN-γ, that they perceive only in vivo or in three-dimensional reaggregates (Fig. 1).
Figure 1.
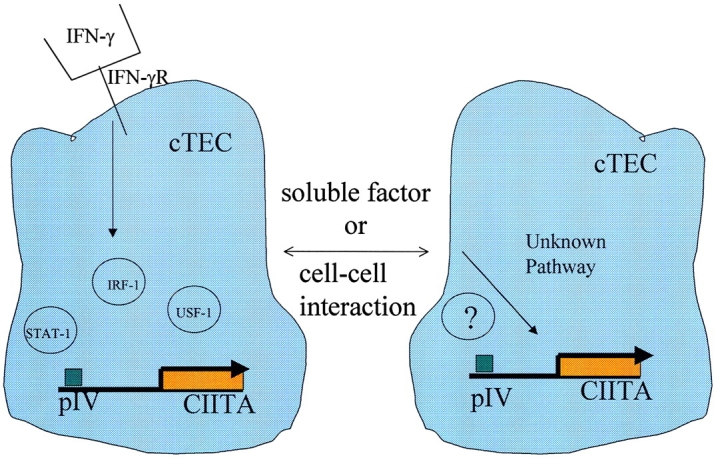
Pathways initiating transcription from pIV of the CIITA gene. Transcription from pIV is initiated in many nonhematopoietic cells, including cTECs, in response to IFN-γ and involves upregulation of the transcription factors STAT-1 and IRF-1. These factors in combination with USF-1 form a complex capable of initiating transcription. A second pathway drives transcription from pIV in cTECs and is responsible for the constitutive expression of MHC class II on these cells in vivo. The stimuli and signal transduction pathway involved in this second pathway are at present unknown, and here we suggest that this stimulus may be provided by either cell–cell contact between cTECs or the release of a short acting soluble factor from cTECs.
Developing thymocytes are one potential cell type that may provide a stimulus to maintain MHC class II on cTECs. However, cTECs isolated from recombination-activating gene (RAG)−/− mice have been shown to express MHC class II and support positive selection of fetal liver cells, implying that neither double-positive thymocytes nor the more mature single-positive thymocytes are required to maintain cTEC MHC class II expression 30. A role for double-negative thymocytes, cells readily detectable in RAG−/− thymi, in maintaining MHC class II expression on cTECs has also been ruled out by in vitro experiments. MHC class II molecules can be detected on epithelial cells from embryonic thymic lobes cultured in the presence of deoxyguanosine 31. This treatment eliminates all thymocytes, including those that are double negative, indicating that these cells are not essential for maintaining cTEC MHC class II expression. Similarly, it has been observed that mesenchymal cells play no role in maintenance of MHC class II on cTECs, as surface expression was not lost in RTOCs containing only purified cTECs and thymocytes 29. These observations suggest that a signal provided either by cell–cell interactions between the cTECs or by a short acting soluble factor produced by cTECs is required to maintain their expression of MHC class II.
One potential candidate for a soluble factor, secreted by thymic epithelial cells, that could induce constitutive MHC class II expression on cTECs is IL-7. However, IL-7 is unlikely to be the sole factor required for maintaining MHC class II expression on cTECs, as MHC class II is expressed at wild-type levels on the surfaces of cTECs isolated from IL-7–deficient mice 30. A role for other soluble factors has not been studied per se; however, the normal development of CD4+ T cells in a wide number of cytokine and cytokine receptor knockout animals, e.g., IL-2, IL-4, IL-15, and IL-15Rβ, implies that MHC class II expression on cTECs is unaffected by their absence 32. These observations provide no evidence to suggest that one specific soluble factor is essential for MHC class II expression on cTECs. However, they do not rule out redundancy among cytokines or a role for alternative or potentially novel cytokines.
The generation of reaggregates from cTECs maintained as a monolayer, i.e., MHC class II–negative cTECs, would allow studies of the role of specific cTEC interactions in inducing and maintaining the expression of MHC class II to be performed. Thus far, studies of this kind have been limited to an analysis of whether such reaggregates are able to support positive selection of CD4+ T cells, which they are not (Anderson, G., personal communication). However, the presence or absence of CD4+ T cell selection is not appropriate as a readout for reexpression of MHC class II on cTECs after culture, as it has been shown that other molecules required for positive selection besides MHC class II are lost when cTECs are cultured on a monolayer of feeder cells 33. The hypothesis that ligation of a cell surface molecule, other than the IFN-γ receptor, can initiate MHC class II expression is not without precedence. It has been reported that expression of MHC class II molecules on human thyroid follicular cells, which are normally HLA-DR negative, can be induced by culturing the cells in the presence of lectins 34, implying that non-APCs can be stimulated to express MHC class II by mechanisms other than IFN-γ.
The observation of Waldburger et al. 21 that in the CIITA pIV−/− mouse, MHC class II expression on cTECs is abrogated, suggests that in cTECs CIITA transcription is initiated from pIV both in response to IFN-γ and in response to an as yet unidentified second stimulus or signal (Fig. 1). It is not clear, however, whether these two pathways are redundant or, in fact, whether both of these pathways operate to maintain MHC class II expression on cTECs in vivo. The role of the IFN-γ pathway is particularly questionable, as few studies have investigated expression of this cytokine in the thymus. In one report, mRNA for IFN-γ was detected in the fetal thymus on days 14–20 of gestation, with levels peaking at day 16 and declining thereafter 35. Whether this is translated into a physiologically significant level of protein in terms of maintaining cTEC MHC class II expression is at present unknown. Additionally, the time frame studied in this report was short, and it is not known whether levels of IFN-γ mRNA persist. Therefore, at present it is difficult to determine the importance of IFN-γ in maintaining MHC class II expression on cTECs. Roles for STAT-1, IRF-1, and USF-1 in regulating the response to IFN-γ have been studied in detail, and this has been discussed above. However, their involvement in the alternative pathway responsible for maintaining constitutive MHC class II expression on cTECs has not been investigated. As a result, studies to define this signaling pathway and the initiating stimulus will be essential for furthering our understanding of the mechanisms of thymocyte development. The existence of a novel pIV-dependent pathway for regulating CIITA and MHC class II expression in cTECs, as implied by the studies of Waldburger et al. 21, indicate that the regulation of CIITA and MHC class II expression is far more complex than had previously been thought.
Acknowledgments
The authors would like to thank Courtney Beers and Drs. Andrew Farr, Sally Clarke, and Peter Gough for insightful comments and critical review of the manuscript.
This work was supported by the Howard Hughes Medical Institute (K. Honey and A. Rudensky) and grants from the National Institutes of Health (to A. Rudensky).
References
- Cresswell P. Assembly, transport, and function of MHC class II molecules. Annu. Rev. Immunol. 1994;12:259–293. doi: 10.1146/annurev.iy.12.040194.001355. [DOI] [PubMed] [Google Scholar]
- Germain R.N. MHC-dependent antigen processing and peptide presentationproviding ligands for T lymphocyte activation. Cell. 1994;76:287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- Ernst B., Lee D.S., Chang J.M., Sprent J., Surh C.D. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- Anderson G., Hare K.J., Jenkinson E.J. Positive selection of thymocytesthe long and winding road. Immunol. Today. 1999;20:463–468. doi: 10.1016/s0167-5699(99)01524-8. [DOI] [PubMed] [Google Scholar]
- Dallman M.J., Mason D.W. Induction of Ia antigens on murine epidermal cells during the rejection of skin allografts. Transplantation. 1983;36:222–224. doi: 10.1097/00007890-198308000-00029. [DOI] [PubMed] [Google Scholar]
- Schattner A. Lymphokines in autoimmunity—a critical review. Clin. Immunol. Immunopathol. 1994;70:177–189. doi: 10.1006/clin.1994.1027. [DOI] [PubMed] [Google Scholar]
- Wojciechowski W., DeSanctis J., Skamene E., Radzioch D. Attenuation of MHC class II expression in macrophages infected with Mycobacterium bovis bacillus Calmette-Guerin involves class II transactivator and depends on the Nramp1 gene. J. Immunol. 1999;163:2688–2696. [PubMed] [Google Scholar]
- Harton J.A., Ting J.P. Class II transactivatormastering the art of major histocompatibility complex expression. Mol. Cell. Biol. 2000;20:6185–6194. doi: 10.1128/mcb.20.17.6185-6194.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldburger J.M., Masternak K., Muhlethaler-Mottet A., Villard J., Peretti M., Landmann S., Reith W. Lessons from the bare lymphocyte syndromemolecular mechanisms regulating MHC class II expression. Immunol. Rev. 2000;178:148–165. doi: 10.1034/j.1600-065x.2000.17813.x. [DOI] [PubMed] [Google Scholar]
- Steimle V., Otten L.A., Zufferey M., Mach B. Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome) Cell. 1993;75:135–146. [PubMed] [Google Scholar]
- Chang C.H., Fontes J.D., Peterlin M., Flavell R.A. Class II transactivator (CIITA) is sufficient for the inducible expression of major histocompatibility complex class II genes. J. Exp. Med. 1994;180:1367–1374. doi: 10.1084/jem.180.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.H., Guerder S., Hong S.C., van Ewijk W., Flavell R.A. Mice lacking the MHC class II transactivator (CIITA) show tissue-specific impairment of MHC class II expression. Immunity. 1996;4:167–178. doi: 10.1016/s1074-7613(00)80681-0. [DOI] [PubMed] [Google Scholar]
- Murphy S.P., Tomasi T.B. Absence of MHC class II antigen expression in trophoblast cells results from a lack of class II transactivator (CIITA) gene expression. Mol. Reprod. Dev. 1998;51:1–12. doi: 10.1002/(SICI)1098-2795(199809)51:1<1::AID-MRD1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Otten L.A., Steimle V., Bontron S., Mach B. Quantitative control of MHC class II expression by the transactivator CIITA. Eur. J. Immunol. 1998;28:473–478. doi: 10.1002/(SICI)1521-4141(199802)28:02<473::AID-IMMU473>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Silacci P., Mottet A., Steimle V., Reith W., Mach B. Developmental extinction of major histocompatibility complex class II gene expression in plasmocytes is mediated by silencing of the transactivator gene CIITA. J. Exp. Med. 1994;180:1329–1336. doi: 10.1084/jem.180.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlethaler-Mottet A., Otten L.A., Steimle V., Mach B. Expression of MHC class II molecules in different cellular and functional compartments is controlled by differential usage of multiple promoters of the transactivator CIITA. EMBO J. 1997;16:2851–2860. doi: 10.1093/emboj/16.10.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskurich J.F., Linhoff M.W., Wang Y., Ting J.P. Two distinct gamma interferon-inducible promoters of the major histocompatibility complex class II transactivator gene are differentially regulated by STAT1, interferon regulatory factor 1, and transforming growth factor beta. Mol. Cell. Biol. 1999;19:431–440. doi: 10.1128/mcb.19.1.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlethaler-Mottet A., Di Berardino W., Otten L.A., Mach B. Activation of the MHC class II transactivator CIITA by interferon-gamma requires cooperative interaction between Stat1 and USF-1. Immunity. 1998;8:157–166. doi: 10.1016/s1074-7613(00)80468-9. [DOI] [PubMed] [Google Scholar]
- Dong Y., Rohn W.M., Benveniste E.N. IFN-gamma regulation of the type IV class II transactivator promoter in astrocytes. J. Immunol. 1999;162:4731–4739. [PubMed] [Google Scholar]
- Cosgrove D., Gray D., Dierich A., Kaufman J., Lemeur M., Benoist C., Mathis D. Mice lacking MHC class II molecules. Cell. 1991;66:1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- Waldburger J.-M., Suter T., Fontana A., Acha-Orbea H., Reith W. Selective abrogation of major histocompatibility complex class II expression on extrahematopoietic cells in mice lacking promoter IV of the class II transactivator gene. J. Exp. Med. 2001;194:393–406. doi: 10.1084/jem.194.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton D.K., Pitts-Meek S., Keshav S., Figari I.S., Bradley A., Stewart T.A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- Huang S., Hendriks W., Althage A., Hemmi S., Bluethmann H., Kamijo R., Vilcek J., Zinkernagel R.M., Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- Matsuyama T., Kimura T., Kitagawa M., Pfeffer K., Kawakami T., Watanabe N., Kundig T.M., Amakawa R., Kishihara K., Wakeham A. Targeted disruption of IRF-1 or IRF-2 results in abnormal type I IFN gene induction and aberrant lymphocyte development. Cell. 1993;75:83–97. [PubMed] [Google Scholar]
- Meraz M.A., White J.M., Sheehan K.C., Bach E.A., Rodig S.J., Dighe A.S., Kaplan D.H., Riley J.K., Greenlund A.C., Campbell D. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- Giunta M., Favre A., Ramarli D., Grossi C.E., Corte G. A novel integrin involved in thymocyte–thymic epithelial cell interactions. J. Exp. Med. 1991;173:1537–1548. doi: 10.1084/jem.173.6.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaud G., De Lerma Barbaro A., Nicolis M., Cestari T., Ramarli D., Riviera A.P., Accolla R.S. Induction of CIITA and modification of in vivo HLA-DR promoter occupancy in normal thymic epithelial cells treated with IFN-gammasimilarities and distinctions with respect to HLA-DR-constitutive B cells. J. Immunol. 1996;156:4254–4258. [PubMed] [Google Scholar]
- Hare K.J., Jenkinson E.J., Anderson G. In vitro models of T cell development. Semin. Immunol. 1999;11:3–12. doi: 10.1006/smim.1998.0151. [DOI] [PubMed] [Google Scholar]
- Anderson G., Jenkinson E.J., Moore N.C., Owen J.J. MHC class II-positive epithelium and mesenchyme cells are both required for T-cell development in the thymus. Nature. 1993;362:70–73. doi: 10.1038/362070a0. [DOI] [PubMed] [Google Scholar]
- Oosterwegel M.A., Haks M.C., Jeffry U., Murray R., Kruisbeek A.M. Induction of TCR gene rearrangements in uncommitted stem cells by a subset of IL-7 producing, MHC class-II-expressing thymic stromal cells. Immunity. 1997;6:351–360. doi: 10.1016/s1074-7613(00)80337-4. [DOI] [PubMed] [Google Scholar]
- Ready A.R., Jenkinson E.J., Kingston R., Owen J.J. Successful transplantation across major histocompatibility barrier of deoxyguanosine-treated embryonic thymus expressing class II antigens. Nature. 1984;310:231–233. doi: 10.1038/310231a0. [DOI] [PubMed] [Google Scholar]
- Porter B.O., Malek T.R. Thymic and intestinal intraepithelial T lymphocyte development are each regulated by the γc-dependent cytokines IL-2, IL-7, and IL-15. Semin. Immunol. 2000;12:465–474. doi: 10.1006/smim.2000.0264. [DOI] [PubMed] [Google Scholar]
- Anderson G., Hare K.J., Platt N., Jenkinson E.J. Discrimination between maintenance- and differentiation-inducing signals during initial and intermediate stages of positive selection. Eur. J. Immunol. 1997;27:1838–1842. doi: 10.1002/eji.1830270803. [DOI] [PubMed] [Google Scholar]
- Pujo-Borrell R., Hanafusa T., Chiovato L., Bottazzo G.F. Lectin-induced expression of DR antigen on human cultured follicular thyroid cells. Nature. 1983;304:71–73. doi: 10.1038/304071a0. [DOI] [PubMed] [Google Scholar]
- Montgomery R.A., Dallman M.J. Semi-quantitative polymerase chain reaction analysis of cytokine and cytokine receptor gene expression during thymic ontogeny. Cytokine. 1997;9:717–726. doi: 10.1006/cyto.1997.0227. [DOI] [PubMed] [Google Scholar]


