Figure 9.
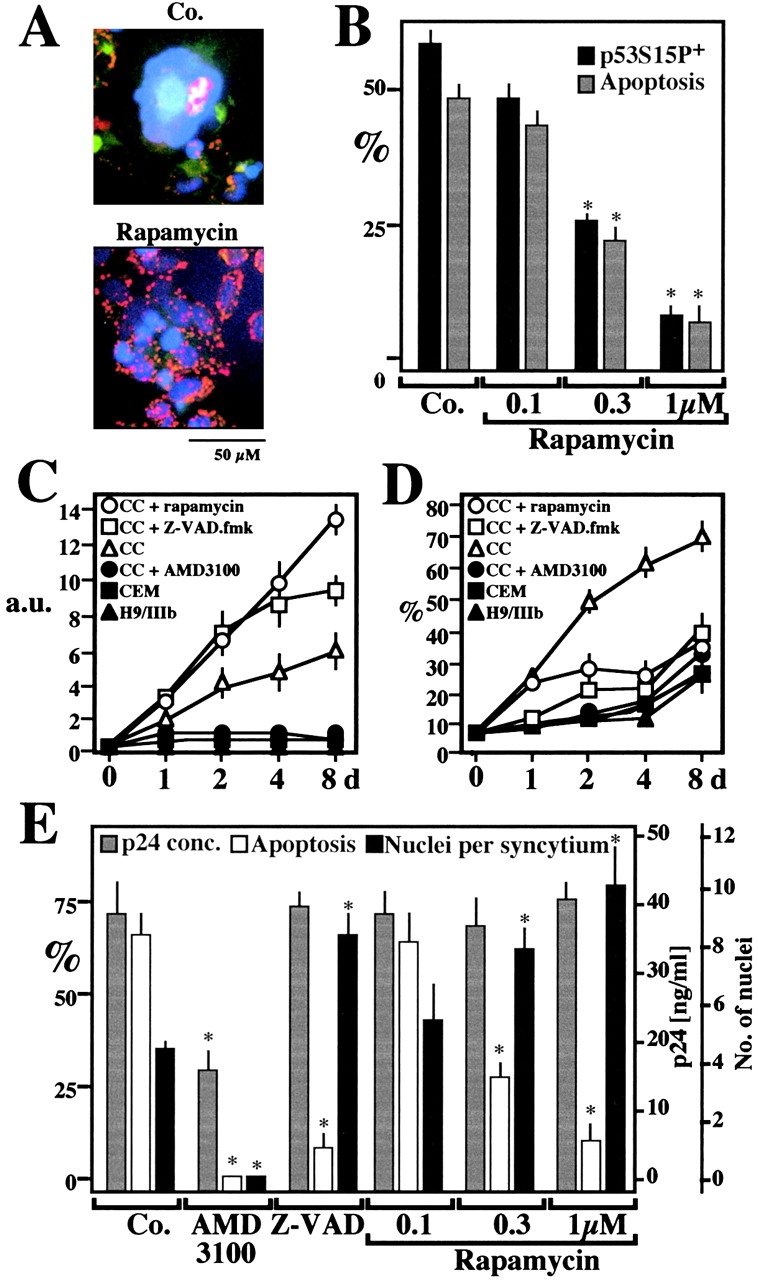
Rapamycin effects on syncytial apoptosis induced by HIV-1 infection. (A) Fluorescence micrographs of HeLa CD4 cells cocultured with HIV-1–infected H9/IIIB cells for 48 h in the absence (Co.) or the presence of 1 μM rapamycin. Cells were stained with Hoechst 33342 and the ΔΨm-sensitive dye JC-1 (as in Fig. 6 A). Primary CD4+ lymphoblasts infected with HIV-1 IIIb are also shown. (B) Rapamycin effects on p53S15P and apoptosis in HeLa CD4 cells cocultured with H9/IIIB cells. p53S15P was determined by immunofluorescence as in Fig. 1 C and apoptosis was measured by staining with JC-1/Hoechst. Values refer to the percentage of syncytia that are p53S15P+ or apoptotic. Asterisks denote significant (P < 0.01) effects of rapamycin, as compared with untreated cocultures. (C and D) Rapamycin-enhanced viability of syncytia associated with apoptosis inhibition. CEM cells stably transfected with a Tat-inducible GFP or HIV-1–infected H9/IIIB cells were cultured alone or cocultured (CC), in the absence or presence of AMD3100 (1 μg/ml), Z-VAD.fmk (50 μM), or rapamycin (1 μM). GFP-dependent fluorescence (arbitrary units, a.u.) was measured to assess the total syncytial mass (C). Parallel cultures were subjected to lysis, staining with propidium iodine, and cytofluorometric determination of the percentage (X ± SEM, n = 3) of hypoploid (sub-G1) nuclei (D). (E) Rapamycin effects on apoptosis of HIV-1 infected primary CD4+ lymphoblasts. 2 d after infection with HIV-IIIB, the inhibitors AMD3100 (which prevents the Env/CD4 interaction, 5 μg/ml), 100 μM Z-VAD.fmk, or rapamycin were added to the cultures. At day 4, the production of p24 (<0.5 ng/ml on day 2) was determined. In addition the frequency of syncytia with condensed nuclei and the number of nuclei per syncytium were determined. Results are mean values ± SEM of three independent experiments. Asterisks denote significant (P < 0.01) effects as compared with control cultures.
