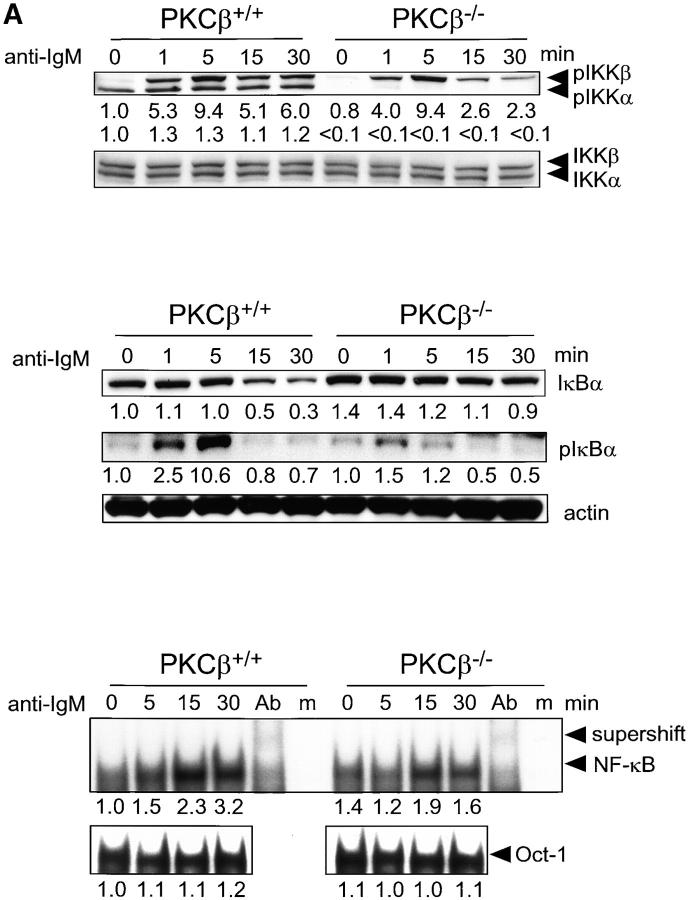
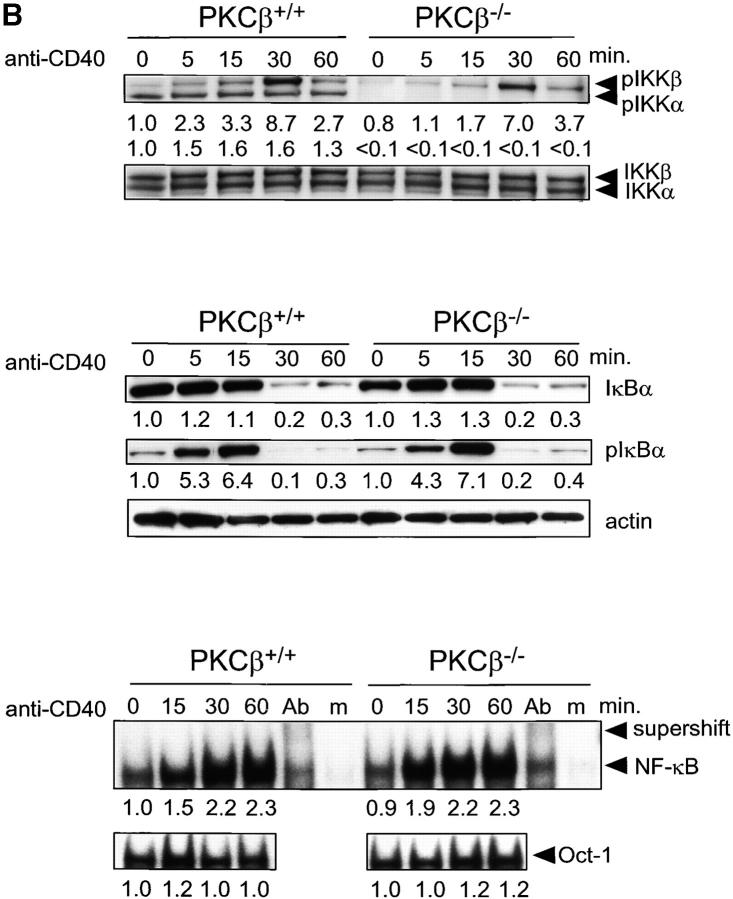
Figure 4.
Impaired activation and NF-κB signaling in PKCβ−/− B cells after BCR cross-linking. Absence of IKKα activation and shortened IKKβ activation in PKCβ−/− B cells. Splenic B cells of PKCβ−/− and PKCβ+/+ mice were stimulated with 20 μg/ml of anti-IgM antibody (A, top panel) or 10 μg/ml anti-CD40 (B, top panel) for the indicated time (min). Western blot analysis of cytoplasmic extracts were done simultaneously with antibodies specific for phospho-Ser180 and phospho-Ser181 of IKKα and IKKβ, respectively (top panel). The membrane was stripped and reprobed with antibodies recognizing nonphosphorylated IKKα and IKKβ. For quantification, band intensities of the phosphorylation site–specific blots were first normalized to the respective signal of unphosphorylated protein and then calculated as fold-change relative to unstimulated PKCβ+/+, which was set to 1.0. Inefficient degradation and phosphorylation of IκBα after IgM cross-linking on PKCβ−/− B cells (A, middle panel) and no alteration in IkBa phosphorylation and degradation after CD40 stimulation (B, middle panel). Splenic B cells were treated as in A. IκBα expression (top) and Ser32 phosphorylation (middle) were analyzed by Western blotting. Equal protein loading was controlled by anti-actin Western blotting (bottom). Quantification was done as in Fig. 2. Reduced DNA binding activity of NF-κB in PKCβ−/− B cells after BCR cross-linking (A, bottom panel) and no alteration after CD40 stimulation (B, bottom panel). EMSA was performed with nuclear extracts of splenic B cells treated as in above. The specificity of NF-κB shift was confirmed by supershift with antibodies for p50, RelA, and c-Rel (Ab) and by using mutant oligonucleotides (m). For quantification, signal intensities were first normalized to the respective signal of an Oct-1 EMSA done with the same nuclear lysates and then calculated as fold-change relative to unstimulated PKCβ+/+, which was set to 1.0.